13.3: The Equilibrium Constant Expression
- Page ID
- 443645
Learning Objectives
- Describe equilibrium constants.
- To write an equilibrium constant expression for any reaction.
- Compare the extent to which a reaction proceeds towards the products using equilibrium constants.
Is a system where the forward and reverse reaction happen at the same time, how do we predict the concentrations, or amounts, or products and reactants when the reaction reaches equilibrium? The rate of the forward and reverse reactions are equal when equilibrium is reached, and the ratio of products to reactants is constant. In equilibrium, we call this ratio the equilibrium constant.
Developing an Equilibrium Constant Expression
In 1864, the Norwegian chemists Cato Guldberg (1836–1902) and Peter Waage (1833–1900) carefully measured the compositions of many reaction systems at equilibrium. They discovered that for any reversible reaction of the general form
\[aA+bB \rightleftharpoons cC+dD \label{10.5.1}\]
where A and B are reactants, C and D are products, and a, b, c, and d are the stoichiometric coefficients in the balanced chemical equation for the reaction, the ratio of the product of the equilibrium concentrations of the products (raised to their coefficients in the balanced chemical equation) to the product of the equilibrium concentrations of the reactants (raised to their coefficients in the balanced chemical equation) is always a constant under a given set of conditions. This relationship is known as the law of mass action and can be stated as follows:
\[K=\dfrac{[C]^c[D]^d}{[A]^a[B]^b} \label{10.5.2}\]
where \(K\) is the equilibrium constant for the reaction. Equation \ref{10.5.1} is called the equilibrium equation, and the right side of Equation \ref{10.5.2} is called the equilibrium constant expression. The relationship shown in Equation \ref{10.5.2} is true for any pair of opposing reactions regardless of the mechanism of the reaction or the number of steps in the mechanism.
There are some rules about writing equilibrium constant expressions that need to be learned:
- The products are on top and the reactants are on the bottom.
- Brackets are used around each species in the reaction to indicate concentration (molarity)
- Concentrations of products are multiplied on the top of the expression. Concentrations of reactants are multiplied together on the bottom.
- Coefficients in the equation become exponents in the equilibrium constant expression. When the coefficient is one, it is not written as an exponent.
- Solids, liquids, and solvents are assigned a value of 1, so their concentrations do not affect the value of K.
The equilibrium constant can vary over a wide range of values. The values of \(K\) shown in Table \(\PageIndex{1}\), for example, vary by 60 orders of magnitude. Because products are in the numerator of the equilibrium constant expression and reactants are in the denominator, values of K greater than \(10^3\) indicate a strong tendency for reactants to form products. In this case, chemists say that equilibrium lies to the right as written, favoring the formation of products. An example is the reaction between \(H_2\) and \(Cl_2\) to produce \(HCl\), which has an equilibrium constant of \(1.6 \times 10^{33}\) at 300 K. Because \(H_2\) is a good reductant and \(Cl_2\) is a good oxidant, the reaction proceeds essentially to completion. In contrast, values of \(K\) less than \(10^{-3}\) indicate that the ratio of products to reactants at equilibrium is very small. That is, reactants do not tend to form products readily, and the equilibrium lies to the left as written, favoring the formation of reactants.
| Reaction | Temperature (K) | Equilibrium Constant (K) |
|---|---|---|
| *Equilibrium constants vary with temperature. The K values shown are for systems at the indicated temperatures. | ||
| \(\ce{S(s) + O2(g) \rightleftharpoons SO2(g)}\) | 300 | \(4.4 \times 10^{53}\) |
| \(\ce{2H2(g) + O2(g) \rightleftharpoons 2H2O (g)}\) | 500 | \(2.4 \times 10^{47}\) |
| \(\ce{H2(g) + Cl2(g) \rightleftharpoons 2 HCl(g)}\) | 300 | \(1.6 \times 10^{33}\) |
| \(\ce{H2(g) + Br2(g) \rightleftharpoons 2HBr(g)}\) | 300 | \(4.1 \times 10^{18}\) |
| \(\ce{2NO(g) + O2(g) \rightleftharpoons 2NO2(g)}\) | 300 | \(4.2 \times 10^{13}\) |
| \(\ce{3H2(g) + N2(g) \rightleftharpoons 2NH3(g)}\) | 300 | \(2.7 \times 10^{8}\) |
| \(\ce{H2(g) + D2(g) \rightleftharpoons 2HD(g)}\) | 100 | \(1.92\) |
| \(\ce{H2(g) + I2(g) \rightleftharpoons 2HI(g)}\) | 300 | \(2.9 \times 10^{−1}\) |
| \(\ce{I2(g) \rightleftharpoons 2I(g)}\) | 800 | \(4.6 \times^{ 10−7}\) |
| \(\ce{Br2(g) \rightleftharpoons 2Br(g)}\) | 1000 | \(4.0 \times 10^{−7}\) |
| \(\ce{Cl2(g) \rightleftharpoons 2Cl (g)}\) | 1000 | \(1.8 \times 10^{−9}\) |
| \(\ce{F2(g) \rightleftharpoons 2F(g)}\) | 500 | \(7.4 \times 10^{−13}\) |
Many reactions have equilibrium constants between 1000 and 0.001 (\(10^3 \ge K \ge 10^{−3}\)), neither very large nor very small. At equilibrium, these systems tend to contain significant amounts of both products and reactants, indicating that there is not a strong tendency to form either products from reactants or reactants from products. An example of this type of system is the reaction of gaseous hydrogen and deuterium, a component of high-stability fiber-optic light sources used in ocean studies, to form HD:
\[H_{2(g)}+D_{2(g)} \rightleftharpoons 2HD_{(g)} \label{10.5.3}\]
The equilibrium constant expression for this reaction is
\[K= \dfrac{[HD]^2}{[H_2][D_2]}\]
with \(K\) varying between 1.9 and 4 over a wide temperature range (100–1000 K). Thus an equilibrium mixture of \(H_2\), \(D_2\), and \(HD\) contains significant concentrations of both product and reactants.
Figure \(\PageIndex{1}\) summarizes the relationship between the magnitude of K and the relative concentrations of reactants and products at equilibrium for a general reaction, written as
\[\text{reactants} \rightleftharpoons \text{products}.\]
When \(K\) is a large number, the concentration of products at equilibrium predominate. This corresponds to an essentially irreversible reaction. Conversely, when \(K\) is a very small number, the reaction produces almost no products as written. Systems for which \(K\) is neither large nor small, the equilibrium will have significant concentrations of both reactants and products.
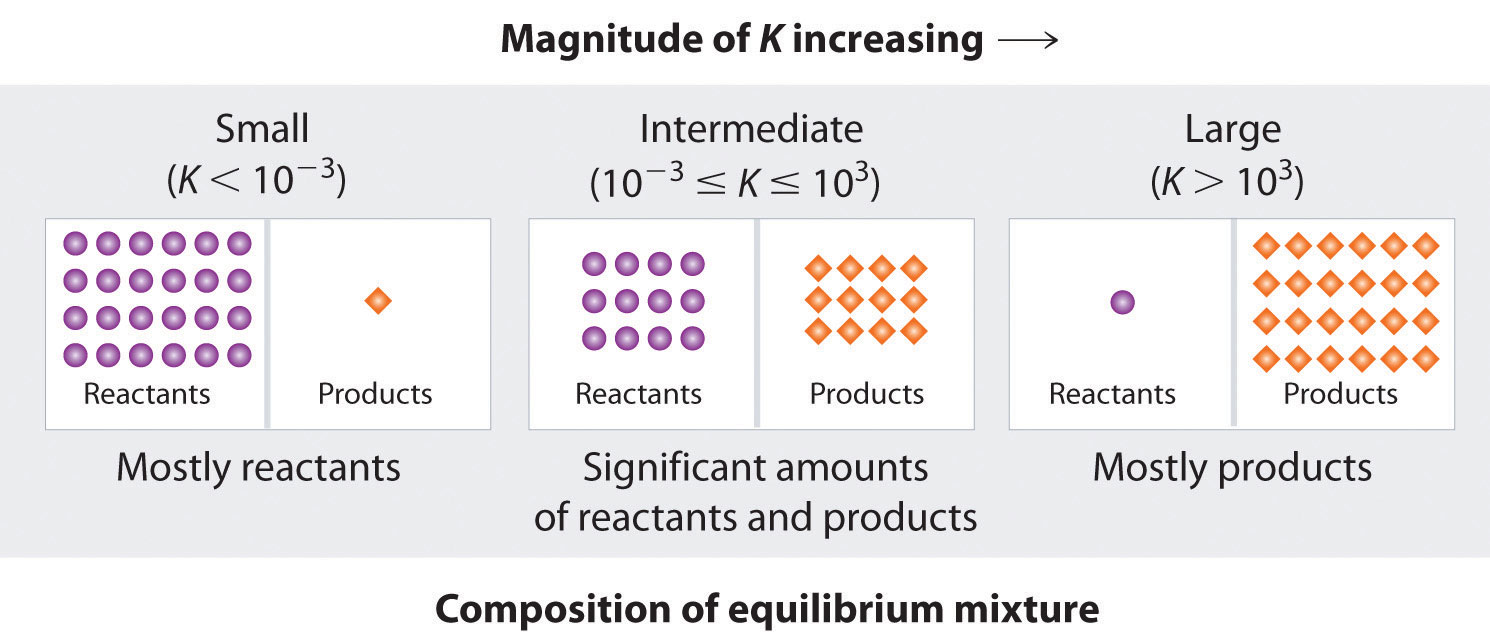
A large value of the equilibrium constant \(K\) means that products predominate at equilibrium; a small value means that reactants predominate at equilibrium.
Example \(\PageIndex{1}\)
Write the equilibrium constant expression for each reaction.
- \(N_{2(g)}+3H_{2(g)} \rightleftharpoons 2NH_{3(g)}\)
- \(CO_{(g)}+\frac{1}{2}O_{2(g)} \rightleftharpoons CO_{2(g)}\)
- \(2CO_{2(g)} \rightleftharpoons 2CO_{(g)}+O_{2(g)}\)
Given: balanced chemical equations
Asked for: equilibrium constant expressions
Strategy:
Refer to Equation \ref{15.2.7}. Place the arithmetic product of the concentrations of the products (raised to their stoichiometric coefficients) in the numerator and the product of the concentrations of the reactants (raised to their stoichiometric coefficients) in the denominator.
Solution:
The only product is ammonia, which has a coefficient of 2. For the reactants, \(N_2\) has a coefficient of 1 and H2 has a coefficient of 3. The equilibrium constant expression is as follows:
\[\dfrac{[NH_3]^2}{[N_2][H_2]^3}\]
The only product is carbon dioxide, which has a coefficient of 1. The reactants are \(CO\), with a coefficient of 1, and \(O_2\), with a coefficient of \(\frac{1}{2}\). Thus the equilibrium constant expression is as follows:
\[\dfrac{[CO_2]}{[CO][O_2]^{1/2}}\]
This reaction is the reverse of the reaction in part b, with all coefficients multiplied by 2 to remove the fractional coefficient for \(O_2\). The equilibrium constant expression is therefore the inverse of the expression in part b, with all exponents multiplied by 2:
\[\dfrac{[CO]^2[O_2]}{[CO_2]^2}\]
Exercise \(\PageIndex{1}\)
Write the equilibrium constant expression for each reaction.
- \(N_2O_{(g)} \rightleftharpoons N_{2(g)}+\frac{1}{2}O_{2(g)}\)
- \(2C_8H_{18(g)}+25O_{2(g)} \rightleftharpoons 16CO_{2(g)}+18H_2O_{(g)}\)
- \(H_{2(g)}+I_{2(g)} \rightleftharpoons 2HI_{(g)}\)
Answer:
- \(K=\dfrac{[N_2][O_2]^{1/2}}{[N_2O]}\)
- \(K=\dfrac{[CO_2]^{16}[H_2O]^{18}}{[C_8H_{18}]^2[O_2]^{25}}\)
- \(K=\dfrac{[HI]^2}{[H_2][I_2]}\)
Example \(\PageIndex{2}\)
Predict which systems at equilibrium will (a) contain essentially only products, (b) contain essentially only reactants, and (c) contain appreciable amounts of both products and reactants.
- \(H_{2(g)}+I_{2(g)} \rightleftharpoons 2HI_{(g)}\;\;\; K_{(700K)}=54\)
- \(2CO_{2(g)} \rightleftharpoons 2CO_{(g)}+O_{2(g)}\;\;\; K_{(1200K)}=3.1 \times 10^{−18}\)
- \(PCl_{5(g)} \rightleftharpoons PCl_{3(g)}+Cl_{2(g)}\;\;\; K_{(613K)}=97\)
- \(2O_{3(g)} \rightleftharpoons 3O_{2(g)} \;\;\; K_{(298 K)}=5.9 \times 10^{55}\)
Given: systems and values of \(K\)
Asked for: composition of systems at equilibrium
Strategy:
Use the value of the equilibrium constant to determine whether the equilibrium mixture will contain essentially only products, essentially only reactants, or significant amounts of both.
Solution:
- Only system 4 has \(K \gg 10^3\), so at equilibrium it will consist of essentially only products.
- System 2 has \(K \ll 10^{−3}\), so the reactants have little tendency to form products under the conditions specified; thus, at equilibrium the system will contain essentially only reactants.
- Both systems 1 and 3 have equilibrium constants in the range \(10^3 \ge K \ge 10^{−3}\), indicating that the equilibrium mixtures will contain appreciable amounts of both products and reactants.
Exercise \(\PageIndex{2}\)
Hydrogen and nitrogen react to form ammonia according to the following balanced chemical equation:
\[3H_{2(g)}+N_{2(g)} \rightleftharpoons 2NH_{3(g)}\]
Values of the equilibrium constant at various temperatures were reported as
- \(K_{25°C} = 3.3 \times 10^8\),
- \(K_{177°C} = 2.6 \times 10^3\), and
- \(K_{327°C} = 4.1\).
At which temperature would you expect to find the highest proportion of \(H_2\) and \(N_2\) in the equilibrium mixture?
Assuming that the reaction rates are fast enough so that equilibrium is reached quickly, at what temperature would you design a commercial reactor to operate to maximize the yield of ammonia?
Answer:
- 327°C, where \(K\) is smallest
- 25°C
The equilibrium constant expression is an equation that we can use to solve for \(K\) or for the concentration of a reactant or product.
Determine the value of \(K\) for the reaction
\[\ce{SO_2} \left( g \right) + \ce{NO_2} \left( g \right) \rightleftharpoons \ce{SO_3} \left( g \right) + \ce{NO} \left( g \right) \nonumber \]
when the equilibrium concentrations are: \(\left[ \ce{SO_2} \right] = 1.20 \: \text{M}\), \(\left[ \ce{NO_2} \right] = 0.60 \: \text{M}\), \(\left[ \ce{NO} \right] = 1.6 \: \text{M}\), and \(\left[ \ce{SO_3} \right] = 2.2 \: \text{M}\).
Solution
Step 1: Write the equilibrium constant expression:
\[K = \dfrac{\left[ \ce{SO_3} \right] \left[ \ce{NO} \right]}{\left[ \ce{SO_2} \right] \left[ \ce{NO_2} \right]} \nonumber \]
Step 2: Substitute in given values and solve:
\[K = \dfrac{\left( 2.2 \right) \left( 1.6 \right)}{\left( 1.20 \right) \left( 0.60 \right)} = 4.9 \nonumber \]
Determining the Equilibrium Expression: https://youtu.be/ZK9cMIWFerY
Summary
- The law of mass action describes a system at equilibrium in terms of the concentrations of the products and the reactants.
- For a system involving one or more gases, either the molar concentrations of the gases or their partial pressures can be used.
- Definition of equilibrium constant in terms of forward and reverse rate constants: \[K=\dfrac{k_f}{k_r} \nonumber\]
- Equilibrium constant expression (law of mass action): \[K=\dfrac{[C]^c[D]^d}{[A]^a[B]^b} \nonumber\]
The ratio of the rate constants for the forward and reverse reactions at equilibrium is the equilibrium constant (K), a unitless quantity. The composition of the equilibrium mixture is therefore determined by the magnitudes of the forward and reverse rate constants at equilibrium. Under a given set of conditions, a reaction will always have the same \(K\). For a system at equilibrium, the law of mass action relates \(K\) to the ratio of the equilibrium concentrations of the products to the concentrations of the reactants raised to their respective powers to match the coefficients in the equilibrium equation. The ratio is called the equilibrium constant expression. When a reaction is written in the reverse direction, \(K\) and the equilibrium constant expression are inverted.

