2.6: Ions - Losing and Gaining Electrons
- Page ID
- 408962
- Define the two types of ions.
Atoms can gain or lose electrons to become ions. When an atom loses an electron it gains a positive charge and is called a cation. When an atom gains an electron it gains a negative charge and is called an anion. The reasons for gaining and losing electrons involve ideas about electron behavior that we will cover in greater detail in a later chapter. Some of the graphics in this section involve the concept of an electron shell. You do not need to understand this concept now, but hopefully the graphics will help with understanding how gaining or losing electrons result in ions with positive or negative charges.
Cations
A neutral sodium atom is likely to achieve an octet in its outermost shell by losing one electron.
\[\ce{Na \rightarrow Na^{+} + e^{-}}\]
The cation produced in this way, Na+, is called the sodium ion to distinguish it from the element. Figure \(\PageIndex{1}\) is a graphical depiction of this process.
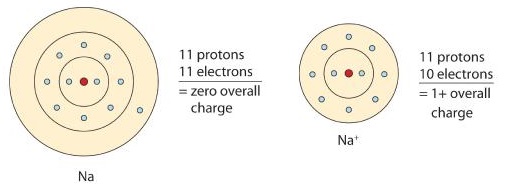
Anions
Some atoms can gain additional electrons. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Negatively charged ions are called anions. Most nonmetals become anions when they make ionic compounds.
\[\ce{e^{-} +Cl -> Cl^{-}}\]
Figure \(\PageIndex{2}\) is a graphical depiction of this process.
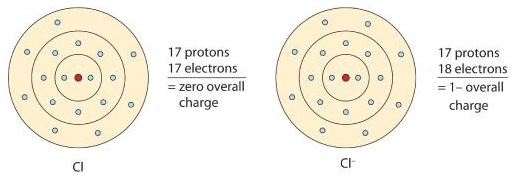
The names for positive and negative ions are pronounced CAT-eye-ons and ANN-eye-ons, respectively.
Contributions & Attributions
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
Henry Agnew (UC Davis)


