In this lecture you will learn the following
- The oxidative addition reactions.
- The reductive elimination reactions.
- Various mechanistic pathways prevalent for these reactions.
Oxidative addition (OA) is a process that adds two anionic ligands e. g. A and B, that originally are a part of a A-B molecule, like in H2 or Me−I, on to a metal center and is of significant importance from the perspective of both synthesis and catalysis. The exact reverse of the same process, in which the two ligands, A and B, are eliminated from the metal center forming back the A−B molecule, is called the reductive elimination (RA). As A and B are anionic X type ligands, the oxidative addition is accompanied by an increase in the coordination number, valence electron count as well as in the formal oxidation state of the metal center by two units. The oxidative addition step may proceed by a variety of pathways. It requires the metal center to be both coordinatively unsaturated and electron deficient.
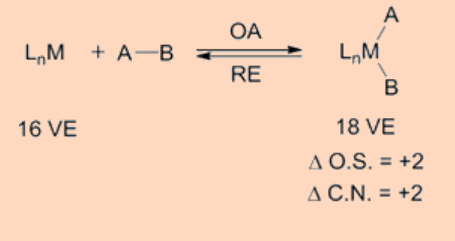
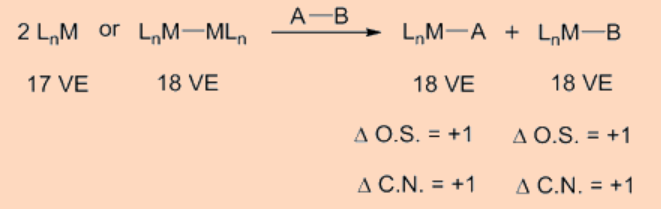
Oxidative addition transfers a single mononuclear metal center having 16 VE to a 18 VE species upon oxidative addition. Another frequently observed pathway is that a 18 VE complex looses a ligand to become a 16 VE species which then undergoes an oxidative addition. Apart from above two types, another possible pathway for oxidative addition proceeds as a binuclear oxidative addition in which each of the two metal centers undergo change in oxidation state, electron count and coordination number by one unit instead of two. This type of a binuclear oxidative addition is observed for a 17 VE metal complex or for a binuclear 18 VE metal complex having a metal−metal bond and, for which the metal has a stable oxidation state at a higher positive oxidation state by one unit.
It is interesting to note that in the oxidative addition the breakage of A−B σ−bond occurs as a result of a net transfer of electrons from the metal center to a σ*−orbital of the A−B bond, thus resulting in the formation of the two new M−A and M−B bonds. The oxidative addition is facilitated by electron rich metal centers having low oxidation state whereas the reductive elimination is facilitated by metal centers in higher oxidation state.
Table \(\PageIndex{1}\). Common types of oxidative addition reactions.
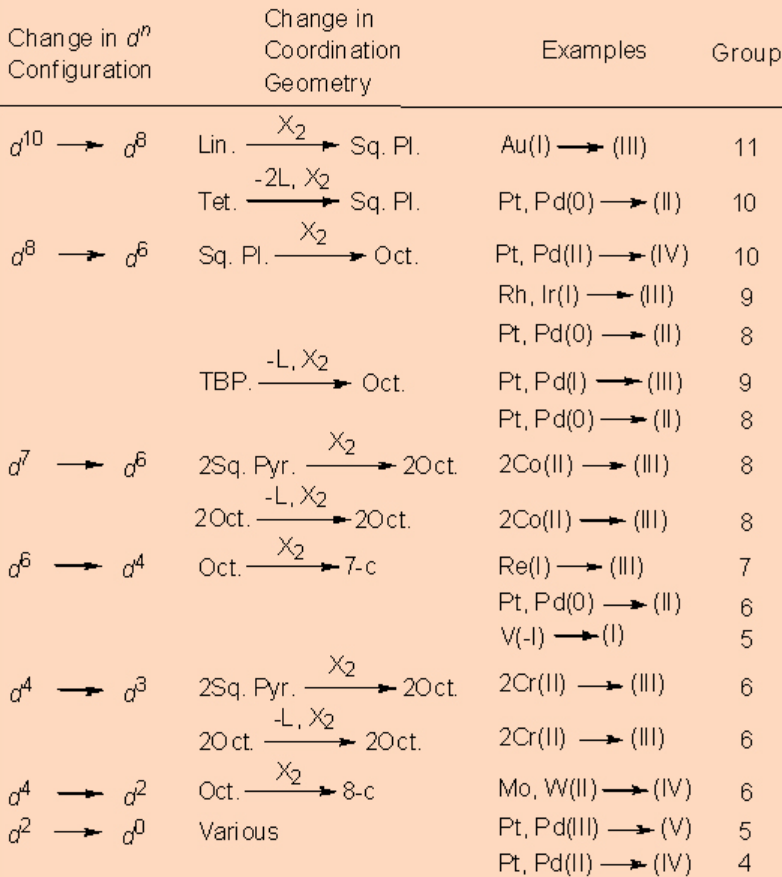
Abbreviations: Lin. = linear, Tet. = tetrahedral, Oct. = octahedral, Sq. Pl. = square planar, TBP = trigonal bipyramidal, Sq. Pyr. = square pyramidal: 7-c, 8-c = 7- and 8-coordinate.
In principle, the oxidative addition is the reverse of reductive elimination, but in practice one may dominate over the other. Thus, the favorability of one over the other is depends on the position of equilibrium, which is further dependent on the stability of the two oxidation states of the metal and on the difference of bond strengths of A−B versus that of the M−A and M−B bonds. For example, metal hydride complexes frequently undergo reductive elimination to give alkanes but rarely an alkane undergoes oxidative addition to give an alkyl hydride complex. Along the same line, alkyl halides frequently undergo oxidative addition to a metal giving metal−alkyl halide complexes but these complexes rarely reductively eliminate to give back alkyl halides. Usually the oxidative addition is more common for 3rd row transition metals because they tend to possess stronger metal ligand bond strengths. The oxidative addition is also favored by strong donor ligands, as they stabilize the higher oxidation state of the metal. The oxidative addition reaction can expand beyond transition metals as observed in the case of the Grignard reagents as well as for some main group elements.
Oxidative addition may proceed by several pathways as discussed below.
Concerted oxidative addition pathway
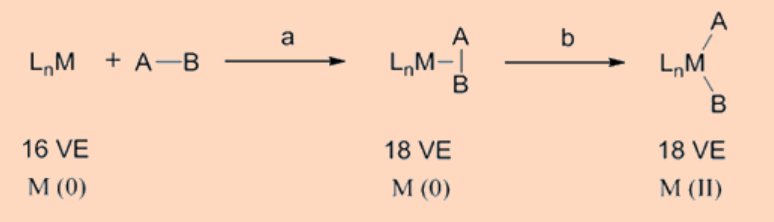
Oxidative addition may proceed by a concerted 3−centered associative mechanism involving the incoming ligand with the metal center. Specifically, the addition proceeds by the formation of a σ−complex upon binding of an incoming ligand say, H2, followed by the cleavage of the H−H bond as a result of the back donation of electrons from the metal to the σ*−orbital of the H−H bond. Such type of addition is common for the H−H, C−H and Si−H bonds. As expected these proceed by two steps (i) the formation of a σ−complex and (ii) the oxidation step. For example, the oxidative addition of H2 to Vaska’s complex (PMe3)2Ir(CO)Cl proceeds by this pathways.
SN2 pathway
This pathway of oxidative addition is operational for the polarized AB type of ligand substrates like the alkyl, acyl, allyl and benzyl halides. In this mechanism, the LnM fragment directly donates electrons to the σ*−orbital of the A−B bond by attacking the least electronegative atom, say A, of the AB molecule and concurrently initiating the elimination of the most electronegative atom of the AB molecule in its anionic form, B−. These reactions proceed via a polar transition state that is accompanied by an inversion of the stereochemistry at the atom of attack by the metal center and are usually accelerated in polar solvents.
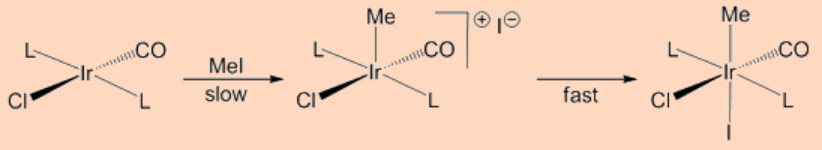
Radical pathway
This type of oxidative addition proceeds via a by radical pathway that generally are vulnerable to the presence of impurities. The radical processes can be of non−chain and chain types. In a non−chain type of mechanism, the metal (M) transfer one electron to the σ*−orbital of the RX bond resulting in the formation of a radical cation M+• and a radical anion RX−•. The generation of the two radical fragments occurs by the way of the elimination of the anion X− from the radical anion RX−• leaving behind the radical R• while the subsequent reaction of X− anion with the radical cation M+• generates the other radical MX• in the course of the reaction. Such type of non−chain type of oxidative addition is observed for the addition of the alkyl halide to Pt(PPh3)3 complexes.
\[\ce{PtL3 ->[fast] PtL2} \nonumber \]
\[\ce{PtL2 + RX -> ^{•}PtL2 + ^{•-}RX ->[slow] ^{•}PtXL2 + ^{•}R} \nonumber \]
\[\ce{^{•}PtXL2 + ^{•}R ->[fast] RPtXL2} \nonumber \]
The other type in this category is the chain radical type reaction that is usually observed for the oxidative addition of EtBr and PhCH2Br to the (PMe3)2Ir(CO)Cl complex. For this process a radical initiator is required and the reaction proceeds along a series of known steps common to a radical process.
\[\ce{R^{•} + IR^{I}Cl(CO)L2 -> RIr^{II•}Cl(CO)L2} \nonumber \]
\[\ce{RIr^{II•}Cl(CO)L2 + RX -> RXIr^{III}Cl(CO)L2 + R^{•}} \nonumber \]
\[\ce{2R^{•} -> R2} \nonumber \]
Ionic pathway
This is kind of pathway for the oxidative addition reaction is common to the addition of hydrogen halides (HX) in its dissociated H+ and X− forms. The ionic pathways are usually of the following two types (i) the ones in which the starting metal complex adds to H+ prior to the addition of the halide X−and (ii) the other type, in which the halide anion X− adds to the starting metal complex first, and then the addition of proton H+ occurs on the metal complex.

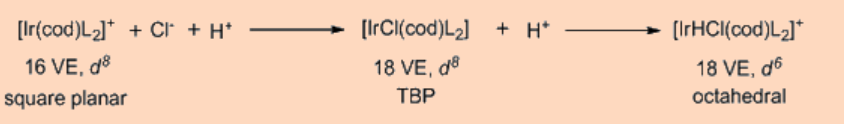
Reductive Elimination
The reductive eliminations are reverse of the oxidative addition reactions and are accompanied by the reduction of the formal oxidation state of the metal and the coordination numbers by two units. The reductive eliminations are commonly observed for d8 systems, like the Ni(II), Pd(II) and Au(III) ions and the d6 systems, like the Pt(IV), Pd(IV), Ir(III) and Rh(III) ions. The reaction may proceed by the elimination of several groups.
\[\ce{L_{n}MRH -> L_{n}M + R-H} \nonumber \]
\[\ce{L_{n}MR2 -> L_{n}M + R-R} \nonumber \]
\[\ce{L_{n}MH(COR) -> L_{n}M + RCHO} \nonumber \]
\[\ce{L_{n}MR(COR) -> L_{n}M + R2CO} \nonumber \]
\[\ce{L_{n}MR(SiR3) -> L_{n}M + R-SiR3} \nonumber \]
Binuclear Reductive Elimination
Similar to what has been observed in the case of binuclear oxidative addition, the binuclear reductive elimination is also observed in some instances. As expected, the oxidation state and the coordination number decrease by one unit in the binuclear reductive elimination pathway.
\[\ce{2MeCH=CHCu(PBu3) ->[heat] MeCH=CHCH=CHMe} \nonumber \]
\[\ce{ArCOMn(CO)5 + HMn(CO)5 -> ArCHO + Mn2(CO)10} \nonumber \]
Problems
1. What kind of metal centers favor oxidative addition?
Ans: Electron rich low valent metal centers.
2. Complete the sentence correctly.
(a) Reductive elimination is frequently observed in coordinatively saturated/unsaturated metal complexes.
(b) Reductive elimination is accompanied by increase/decrease in the oxidation state of the metal.
(c) Oxidative addition is accompanied by increase/decrease in the coordination number of the metal
Ans:
(a) Saturated.
(b) Decrease in the oxidation state by two units.
(c) Increase in the coordination number by two units
3. State the various mechanistic pathways involved in oxidative addition reactions.
Ans: Concerted oxidative addition, SN2 mechanism, radical and ionic mechanism.
4. Complete the reaction.
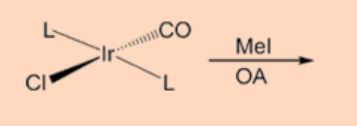
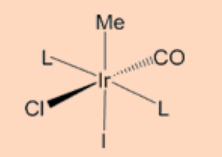
Ans:
Self Assessment test
1. What kind of metal centers favor reductive elimination?
Ans: Electron deficient high valent metal centers
2. Complete the sentence correctly.
(a) Oxidative addition is frequently observed in coordinatively saturated/unsaturated metal complexes.
(b) Oxidative addition is accompanied by increase/decrease in the oxidation state of the metal.
(c) Reductive elimination is accompanied by increase/decrease in the coordination number of the metal.
Ans:
(a) Unsaturated.
(b) Increase in the oxidation state by two units.
(c) Decrease in the coordination numbers by two units.
3. How does the geometry of the square planar complexes change upon oxidative addition reactions?
Ans: Square planar to octahedral.
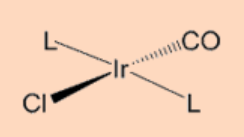
4. Complete the reaction.
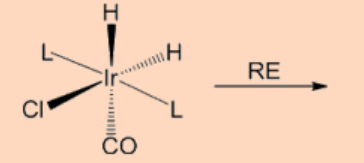
Ans:
Summary
The oxidative addition and the reductive elimination reactions are like the observe and reverse of the same coin. The oxidative addition is generally observed for metal centers with low oxidation state and is usually accompanied by the increase in the oxidation state, the valence electron count and the coordination number of the metal by two units. Being opposite, the reductive elimination is seen in the case of the metal centers with higher oxidation state and is accompanied by the decrease in the oxidation state, the valence electron count and the coordination number of the metal by two units. The oxidative addition may proceed by a variety of pathways that involve concerted, ionic and the radical based mechanisms. More interestingly, the oxidative addition and reductive elimination reactions are not solely restricted to the mononuclear metal complexes but can also be observed for the binuclear complexes.












