5.7: End of Chapter Problems
- Page ID
- 369411
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- What does it mean to say an equation is balanced? Why is it important for an equation to be balanced?
- Answer
- An equation is balanced when the same number of each element is represented on the reactant and product sides. Equations must be balanced to accurately reflect the law of conservation of matter.
- Consider molecular, complete ionic, and net ionic equations. What is the difference between these types of equations? In what circumstance would the complete and net ionic equations for a reaction be identical?
- Balance the following equations:
- \(\ce{PCl5}(s)+\ce{H2O}(l)\rightarrow \ce{POCl3}(l)+\ce{HCl}(aq)\)
- \(\ce{Cu}(s)+\ce{HNO3}(aq)\rightarrow \ce{Cu(NO3)2}(aq)+\ce{H2O}(l)+\ce{NO}(g)\)
- \(\ce{H2}(g)+\ce{I2}(s)\rightarrow \ce{HI}(s)\)
- \(\ce{Fe}(s)+\ce{O2}(g)\rightarrow \ce{Fe2O3}(s)\)
- \(\ce{Na}(s)+\ce{H2O}(l)\rightarrow \ce{NaOH}(aq)+\ce{H2}(g)\)
- \(\ce{(NH4)2Cr2O7}(s)\rightarrow \ce{Cr2O3}(s)+\ce{N2}(g)+\ce{H2O}(g)\)
- \(\ce{P4}(s)+\ce{Cl2}(g)\rightarrow \ce{PCl3}(l)\)
- \(\ce{PtCl4}(s)\rightarrow \ce{Pt}(s)+\ce{Cl2}(g)\)
- Answer
- a. \(\ce{PCl5}(s)+\ce{H2O}(l)\rightarrow \ce{POCl3}(l)+\ce{2HCl}(aq)\);b. \(\ce{3Cu}(s)+\ce{8HNO3}(aq)\rightarrow \ce{3Cu(NO3)2}(aq)+\ce{4H2O}(l)+\ce{2NO}(g)\); c. \(\ce{H2}(g)+\ce{I2}(s)\rightarrow \ce{2HI}(s)\) ; d. \(\ce{4Fe}(s)+\ce{3O2}(g)\rightarrow \ce{2Fe2O3}(s)\); e. \(\ce{2Na}(s)+\ce{2H2O}(l)\rightarrow \ce{2NaOH}(aq)+\ce{H2}(g)\); f. \(\ce{(NH4)2Cr52O7}(s)\rightarrow \ce{Cr2O3}(s)+\ce{N2}(g)+\ce{4H2O}(g)\); g. \(\ce{P4}(s)+\ce{6Cl2}(g)\rightarrow \ce{4PCl3}(l)\) ; h. \(\ce{PtCl4}(s)\rightarrow \ce{Pt}(s)+\ce{2Cl2}(g)\)
- Balance the following equations:
- \(\ce{Ag}(s)+\ce{H2S}(g)+\ce{O2}(g)\rightarrow \ce{Ag2S}(s)+\ce{H2O}(l)\)
- \(\ce{P4}(s)+\ce{O2}(g)\rightarrow \ce{P4O10}(s)\)
- \(\ce{Pb}(s)+\ce{H2O}(l)+\ce{O2}(g)\rightarrow \ce{Pb(OH)2}(s)\)
- \(\ce{Fe}(s)+\ce{H2O}(l)\rightarrow \ce{Fe3O4}(s)+\ce{H2}(g)\)
- \(\ce{Sc2O3}(s)+\ce{SO3}(l)\rightarrow \ce{Sc2(SO4)3}(s)\)
- \(\ce{Ca3(PO4)2}(aq)+\ce{H3PO4}(aq)\rightarrow \ce{Ca(H2PO4)2}(aq)\)
- \(\ce{Al}(s)+\ce{H2SO4}(aq)\rightarrow \ce{Al2(SO4)3}(s)+\ce{H2}(g)\)
- \(\ce{TiCl4}(s)+\ce{H2O}(g)\rightarrow \ce{TiO2}(s)+\ce{HCl}(g)\)
- Answer
- a. \(\ce{4Ag}(s)+\ce{2H2S}(g)+\ce{O2}(g)\rightarrow \ce{2Ag2S}(s)+\ce{2H2O}(l)\); b. \(\ce{P4}(s)+\ce{5O2}(g)\rightarrow \ce{P4O10}(s)\); c. \(\ce{2Pb}(s)+\ce{2H2O}(l)+\ce{O2}(g)\rightarrow \ce{2Pb(OH)2}(s)\); d. \(\ce{3Fe}(s)+\ce{4H2O}(l)\rightarrow \ce{Fe3O4}(s)+\ce{4H2}(g)\); e. \(\ce{Sc2O3}(s)+\ce{3SO3}(l)\rightarrow \ce{Sc2(SO4)3}(s)\); f. \(\ce{Ca3(PO4)2}(aq)+\ce{4H3PO4}(aq)\rightarrow \ce{3Ca(H2PO4)2}(aq)\); g. \(\ce{2Al}(s)+\ce{3H2SO4}(aq)\rightarrow \ce{Al2(SO4)3}(s)+\ce{3H2}(g)\); h. \(\ce{TiCl4}(s)+\ce{2H2O}(g)\rightarrow \ce{TiO2}(s)+\ce{4HCl}(g)\)
- Write a balanced molecular equation describing each of the following chemical reactions.
- Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas.
- Gaseous butane, C4H10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor.
- Aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride.
- Water vapor reacts with sodium metal to produce solid sodium hydroxide and hydrogen gas.
- Answer
- a. \(\ce{CaCO3}(s)\rightarrow \ce{CaO}(s)+\ce{CO2}(g)\); b. \(\ce{2C4H10}(g)+\ce{13O2}(g)\rightarrow \ce{8CO2}(g)+\ce{10H2O}(g)\); c. \(\ce{MgCl2}(aq)+\ce{2NaOH}(aq)\rightarrow \ce{Mg(OH)2}(s)+\ce{2NaCl}(aq)\); d. \(\ce{2H2O}(g)+\ce{2Na}(s)\rightarrow \ce{2NaOH}(s)+\ce{H2}(g)\)
- Write a balanced equation describing each of the following chemical reactions.
- Solid potassium chlorate, KClO3, decomposes to form solid potassium chloride and diatomic oxygen gas.
- Solid aluminum metal reacts with solid diatomic iodine to form solid Al2I6.
- When solid sodium chloride is added to aqueous sulfuric acid, hydrogen chloride gas and aqueous sodium sulfate are produced.
- Aqueous solutions of phosphoric acid and potassium hydroxide react to produce aqueous potassium dihydrogen phosphate and liquid water.
- Colorful fireworks often involve the decomposition of barium nitrate and potassium chlorate and the reaction of the metals magnesium, aluminum, and iron with oxygen.
- Write the formulas of barium nitrate and potassium chlorate.
- The decomposition of solid potassium chlorate leads to the formation of solid potassium chloride and diatomic oxygen gas. Write an equation for the reaction.
- The decomposition of solid barium nitrate leads to the formation of solid barium oxide, diatomic nitrogen gas, and diatomic oxygen gas. Write an equation for the reaction.
- Write separate equations for the reactions of the solid metals magnesium, aluminum, and iron with diatomic oxygen gas to yield the corresponding metal oxides. (Assume the iron oxide contains Fe3+ ions.)
- Answer
- a. Ba(NO3)2, KClO3; b. \(\ce{2KClO3}(s)\rightarrow \ce{2KCl}(s)+\ce{3O2}(g)\); c. \(\ce{2Ba(NO3)2}(s)\rightarrow \ce{2BaO}(s)+\ce{2N2}(g)+\ce{5O2}(g)\); d. \(\ce{2Mg}(s)+\ce{O2}(g)\rightarrow \ce{2MgO}(s)\) ; \(\ce{4Al}(s)+\ce{3O2}(g)\rightarrow \ce{2Al2O3}(g)\); \(\ce{4Fe}(s)+\ce{3O2}(g)\rightarrow \ce{2Fe2O3}(s)\)
- Fill in the blank with a single chemical formula for a covalent compound that will balance the equation:
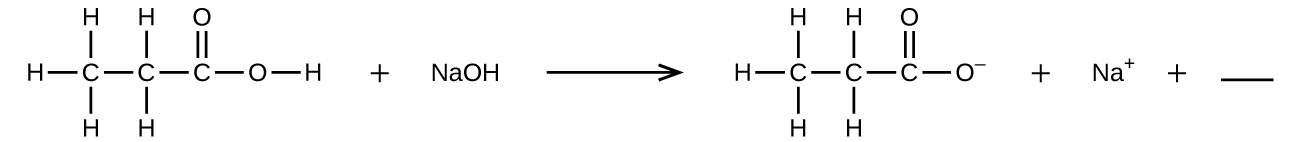
- Aqueous hydrogen fluoride (hydrofluoric acid) is used to etch glass and to analyze minerals for their silicon content. Hydrogen fluoride will also react with sand (silicon dioxide).
- Write an equation for the reaction of solid silicon dioxide with hydrofluoric acid to yield gaseous silicon tetrafluoride and liquid water.
- The mineral fluorite (calcium fluoride) occurs extensively in Illinois. Solid calcium fluoride can also be prepared by the reaction of aqueous solutions of calcium chloride and sodium fluoride, yielding aqueous sodium chloride as the other product. Write complete and net ionic equations for this reaction.
- Answer
- a. 4HF(aq)+SiO2(s)→SiF4(g)+2H2O(l)4HF(aq)+SiO2(s)→SiF4(g)+2H2O(l); b. complete ionic equation: 2Na+(aq)+2F−(aq)+Ca2+(aq)+2Cl−(aq)→CaF2(s)+2Na+(aq)+2Cl−(aq)2Na+(aq)+2F−(aq)+Ca2+(aq)+2Cl−(aq)→CaF2(s)+2Na+(aq)+2Cl−(aq), net ionic equation: 2F−(aq)+Ca2+(aq)→CaF2(s)
- \(\ce{4HF}(aq)+\ce{SiO2}(s)\rightarrow \ce{SiF4}(g)+\ce{2H2O}(l)\);
- complete ionic equation: \(\ce{2Na+}(aq)+\ce{2F-}(aq)+\ce{Ca^2+}(aq)+\ce{2Cl-}(aq)\rightarrow \ce{CaF2}(s)+\ce{2Na+}(aq)+\ce{2Cl-}(aq)\), net ionic equation: \(\ce{2F-}(aq)+\ce{Ca^2+}(aq)\rightarrow \ce{CaF2}(s)\)
- A novel process for obtaining magnesium from sea water involves several reactions. Write a balanced chemical equation for each step of the process.
- The first step is the decomposition of solid calcium carbonate from seashells to form solid calcium oxide and gaseous carbon dioxide.
- The second step is the formation of solid calcium hydroxide as the only product from the reaction of the solid calcium oxide with liquid water.
- Solid calcium hydroxide is then added to the seawater, reacting with dissolved magnesium chloride to yield solid magnesium hydroxide and aqueous calcium chloride.
- The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water.
- Finally, the magnesium chloride is melted and electrolyzed to yield liquid magnesium metal and diatomic chlorine gas.
- From the balanced molecular equations, write the complete ionic and net ionic equations for the following:
- \(\ce{K2C2O4}(aq)+\ce{Ba(OH)2}(aq)\rightarrow \ce{2KOH}(aq)+\ce{BaC2O2}(s)\)
- \(\ce{Pb(NO3)2}(aq)+\ce{H2SO4}(aq)\rightarrow \ce{PbSO4}(s)+\ce{2HNO3}(aq)\)
- \(\ce{CaCO3}(s)+\ce{H2SO4}(aq)\rightarrow \ce{CaSO4}(s)+\ce{CO2}(g)+\ce{H2O}(l)\)
- Answer
- a. \[\ce{2K+}(aq)+\ce{C2O4^2-}(aq)+\ce{Ba^2+}(aq)+\ce{2OH-}(aq)\rightarrow \ce{2K+}(aq)+\ce{2OH-}(aq)+\ce{BaC2O4}(s)\hspace{20px}\ce{(complete)}\] \[\ce{Ba^2+}(aq)+\ce{C2O4^2-}(aq)\rightarrow \ce{BaC2O4}(s)\hspace{20px}\ce{(net)}\]; b. \[\ce{Pb^2+}(aq)+\ce{2NO3-}(aq)+\ce{2H+}(aq)+\ce{SO4^2-}(aq)\rightarrow \ce{PbSO4}(s)+\ce{2H+}(aq)+\ce{2NO3-}(aq)\hspace{20px}\ce{(complete)}\]\[\ce{Pb^2+}(aq)+\ce{SO4^2-}(aq)\rightarrow \ce{PbSO4}(s)\hspace{20px}\ce{(net)}\]; c. \[\ce{CaCO3}(s)+\ce{2H+}(aq)+\ce{SO4^2-}(aq)\rightarrow \ce{CaSO4}(s)+\ce{CO2}(g)+\ce{H2O}(l)\hspace{20px}\ce{(complete)}\]\[\ce{CaCO3}(s)+\ce{2H+}(aq)+\ce{SO4^2-}(aq)\rightarrow \ce{CaSO4}(s)+\ce{CO2}(g)+\ce{H2O}(l)\hspace{20px}\ce{(net)}\];
- Use the following equations to answer the next five questions:
- \(\ce{H2O}(s)\rightarrow \ce{H2O}(l)\)
- \(\ce{Na+}(aq)+\ce{Cl-}(aq)\ce{Ag+}(aq)+\ce{NO3-}(aq) \rightarrow \ce{AgCl}(s)+\ce{Na+}(aq)+\ce{NO3-}(aq)\)
- \(\ce{CH3OH}(g)+\ce{O2}(g)\rightarrow \ce{CO2}(g)+\ce{H2O}(g)\)
- \(\ce{2H2O}(l)\rightarrow \ce{2H2}(g)+\ce{O2}(g)\)
- \(\ce{H+}(aq)+\ce{OH-}(aq)\rightarrow \ce{H2O}(l)\)
- Which equation describes a physical change?
- Which equation identifies the reactants and products of a combustion reaction?
- Which equation is not balanced?
- Which is a net ionic equation?
- Answer
- a.) i; b.) iii; c.) iii; d.) v
13. Indicate what type, or types, of reaction each of the following represents:
- \(\ce{Ca}(s)+\ce{Br2}(l)\rightarrow \ce{CaBr2}(s)\)
- \(\ce{Ca(OH)2}(aq)+\ce{2HBr}(aq)\rightarrow \ce{CaBr2}(aq)+\ce{2H2O}(l)\)
- \(\ce{C6H12}(l)+\ce{9O2}(g)\rightarrow \ce{6CO2}(g)+\ce{6H2O}(g)\)
- Answer
- a. oxidation-reduction (addition); b. acid-base (neutralization); c.oxidation-reduction (combustion)
- Indicate what type, or types, of reaction each of the following represents:
- \(\ce{H2O}(g)+\ce{C}(s)\rightarrow \ce{CO}(g)+\ce{H2}(g)\)
- \(\ce{2KClO3}(s)\rightarrow \ce{2KCl}(s)+\ce{3O2}(g)\)
- \(\ce{Al(OH)3}(aq)+\ce{3HCl}(aq)\rightarrow \ce{AlBr3}(aq)+\ce{3H2O}(l)\)
- \(\ce{Pb(NO3)2}(aq)+\ce{H2SO4}(aq)\rightarrow \ce{PbSO4}(s)+\ce{2HNO3}(aq)\)
- Silver can be separated from gold because silver dissolves in nitric acid while gold does not. Is the dissolution of silver in nitric acid an acid-base reaction or an oxidation-reduction reaction? Explain your answer.
- Answer
- It is an oxidation-reduction reaction because the oxidation state of the silver changes during the reaction.
- Determine the oxidation states of the elements in the following compounds:
- NaI
- GdCl3
- LiNO3
- H2Se
- Mg2Si
- RbO2, rubidium superoxide
- HF
- Determine the oxidation states of the elements in the compounds listed. None of the oxygen-containing compounds are peroxides or superoxides.
- H3PO4
- Al(OH)3
- SeO2
- KNO2
- In2S3
- P4O6
- Answer
- a. H +1, P +5, O −2; b. Al +3, H +1, O −2; c. Se +4, O −2; d. K +1, N +3, O −2; e. In +3, S −2; f. P +3, O −2
- Determine the oxidation states of the elements in the compounds listed. None of the oxygen-containing compounds are peroxides or superoxides.
- H2SO4
- Ca(OH)2
- BrOH
- ClNO2
- TiCl4
- NaH
- Answer
- a. H1+, O2-, S6+; b. H1+, O2-, Ca+2 ; c. H1+, O2-, Br1+ ;d. O2-, Cl1-, N5+ ;e. Cl1-, Ti4+ ; f. H1+, Na1-
- Classify the following as acid-base reactions or oxidation-reduction reactions:
- \(\ce{Na2S}(aq)+\ce{2HCl}(aq)\rightarrow \ce{2NaCl}(aq)+\ce{H2S}(g)\)
- \(\ce{2Na}(s)+\ce{2HCl}(aq)\rightarrow \ce{2NaCl}(aq)+\ce{H2}(g)\)
- \(\ce{Mg}(s)+\ce{Cl2}(g)\rightarrow \ce{MgCl2}(s)\)
- \(\ce{MgO}(s)+\ce{2HCl}(aq)\rightarrow \ce{MgCl2}(aq)+\ce{H2O}(l)\)
- \(\ce{K3P}(s)+\ce{2O2}(g)\rightarrow \ce{K3PO4}(s)\)
- \(\ce{3KOH}(aq)+\ce{H3PO4}(aq)\rightarrow \ce{K3PO4}(aq)+\ce{3H2O}(l)\)
- Answer
- a. acid-base; b. oxidation-reduction: Na is oxidized, H+ is reduced; c. oxidation-reduction: Mg is oxidized, Cl2 is reduced; d. acid-base; e. oxidation-reduction: P3− is oxidized, O2 is reduced; f. acid-base
- Identify the atoms that are oxidized and reduced, the change in oxidation state for each, and the oxidizing and reducing agents in each of the following equations:
- \(\ce{Mg}(s)+\ce{NiCl2}(aq)\rightarrow \ce{MgCl2}(aq)+\ce{Ni}(s)\)
- \(\ce{PCl3}(l)+\ce{Cl2}(g)\rightarrow \ce{PCl5}(s)\)
- \(\ce{C2H4}(g)+\ce{3O2}(g)\rightarrow \ce{2CO2}(g)+\ce{2H2O}(g)\)
- \(\ce{Zn}(s)+\ce{H2SO4}(aq)\rightarrow \ce{ZnSO4}(aq)+\ce{H2}(g)\)
- \(\ce{2K2S2O3}(s)+\ce{I2}(s)\rightarrow \ce{K2S4O6}(s)+\ce{2KI}(s)\)
- \(\ce{3Cu}(s)+\ce{8HNO3}(aq)\rightarrow\ce{3Cu(NO3)2}(aq)+\ce{2NO}(g)+\ce{4H2O}(l)\)
- Complete and balance the following acid-base equations:
- HCl gas reacts with solid Ca(OH)2(s).
- A solution of Sr(OH)2 is added to a solution of HNO3.
- Answer
- a. \(\ce{2HCl}(g)+\ce{Ca(OH)2}(s)\rightarrow \ce{CaCl2}(s)+\ce{2H2O}(l)\); b. \(\ce{Sr(OH)2}(aq)+\ce{2HNO3}(aq)\rightarrow \ce{Sr(NO3)2}(aq)+\ce{2H2O}(l)\)
- Complete and balance the following acid-base equations:
- A solution of HClO4 is added to a solution of LiOH.
- Aqueous H2SO4 reacts with NaOH.
- Ba(OH)2 reacts with HF gas.
- Complete and balance the following oxidation-reduction reactions, which give the highest possible oxidation state for the oxidized atoms.
- \(\ce{Al}(s)+\ce{F2}(g)\rightarrow\)
- \(\ce{Al}(s)+\ce{CuBr2}(aq)\rightarrow\) (single displacement)
- \(\ce{P4}(s)+\ce{O2}(g)\rightarrow \)
- \(\ce{Ca}(s)+\ce{H2O}(l)\rightarrow \) (products are a strong base and a diatomic gas)
- Answer
- a.\(\ce{2Al}(s)+\ce{3F2}(g)\rightarrow \ce{2AlF3}(s)\); b. \(\ce{2Al}(s)+\ce{3CuBr2}(aq)\rightarrow \ce{3Cu}(s)+\ce{2AlBr3}(aq)\); c. \(\ce{P4}(s)+\ce{5O2}(g)\rightarrow \ce{P4O10}(s)\); d. \(\ce{Ca}(s)+\ce{2H2O}(l)\rightarrow \ce{Ca(OH)2}(aq)+\ce{H2}(g)\)
- Complete and balance the following oxidation-reduction reactions, which give the highest possible oxidation state for the oxidized atoms.
- \(\ce{K}(s)+\ce{H2O}(l)\rightarrow \)
- \(\ce{Ba}(s)+\ce{HBr}(aq)\rightarrow \)
- \(\ce{Sn}(s)+\ce{I2}(s)\rightarrow \)
- Complete and balance the equations for the following acid-base neutralization reactions. If water is used as a solvent, write the reactants and products as aqueous ions. In some cases, there may be more than one correct answer, depending on the amounts of reactants used.
- \(\ce{Mg(OH)2}(s)+\ce{HClO4}(aq)\rightarrow \)
- \(\ce{SO3}(g)+\ce{H2O}(l)\rightarrow \) (assume an excess of water and that the product dissolves)
- \(\ce{SrO}(s)+\ce{H2SO4}(l)\rightarrow \)
- Answer
- a.\(\ce{Mg(OH)2}(s)+\ce{2HClO4}(aq)\rightarrow \ce{Mg^2+}(aq)+\ce{2ClO4-}(aq)+\ce{2H2O}(l)\) ; b. \(\ce{SO3}(g)+\ce{2H2O}(l)\rightarrow \ce{H3O+}(aq)+\ce{HSO4-}(aq)\), (a solution of H2SO4); c. \(\ce{SrO}(s)+\ce{H2SO4}(l)\rightarrow \ce{SrSO4}(s)+\ce{H2O}\)
- When heated to 700–800 °C, diamonds, which are pure carbon, are oxidized by atmospheric oxygen. (They burn!) Write the balanced equation for this reaction.
- The military has experimented with lasers that produce very intense light when fluorine combines explosively with hydrogen. What is the balanced equation for this reaction?
- Answer
- \(\ce{H2}(g)+\ce{F2}(g)\rightarrow \ce{2HF}(g)\)
- Write the molecular, total ionic, and net ionic equations for the following reactions:
- \(\ce{Ca(OH)2}(aq)+\ce{HC2H3O2}(aq)\rightarrow \)
- \(\ce{H3PO4}(aq)+\ce{CaCl2}(aq)\rightarrow \)
- Great Lakes Chemical Company produces bromine, Br2, from bromide salts such as NaBr, in Arkansas brine by treating the brine with chlorine gas. Write a balanced equation for the reaction of NaBr with Cl2.
- Answer
- \(\ce{2NaBr}(aq)+\ce{Cl2}(g)\rightarrow \ce{2NaCl}(aq)+\ce{Br2}(l)\)
- In a common experiment in the general chemistry laboratory, magnesium metal is heated in air to produce MgO. MgO is a white solid, but in these experiments it often looks gray, due to small amounts of Mg3N2, a compound formed as some of the magnesium reacts with nitrogen. Write a balanced equation for each reaction.
- Lithium hydroxide may be used to absorb carbon dioxide in enclosed environments, such as manned spacecraft and submarines. Write an equation for the reaction that involves 2 mol of LiOH per 1 mol of CO2. (Hint: Water is one of the products.)
- Answer
- \(\ce{2LiOH}(aq)+\ce{CO2}(g)\rightarrow \ce{Li2CO3}(aq)+\ce{H2O}(l)\)
- Calcium propionate is sometimes added to bread to retard spoilage. This compound can be prepared by the reaction of calcium carbonate, CaCO3, with propionic acid, C2H5CO2H, which has properties similar to those of acetic acid. Write the balanced equation for the formation of calcium propionate.
- Complete and balance the equations of the following reactions, each of which could be used to remove hydrogen sulfide from natural gas:
- \(\ce{Ca(OH)2}(s)+\ce{H2S}(g) \rightarrow\)
- \(\ce{Na2CO3}(aq)+\ce{H2S}(g)\rightarrow \)
- Answer
- a.\(\ce{Ca(OH)2}(s)+\ce{H2S}(g)\rightarrow \ce{CaS}(s)+\ce{2H2O}(l)\); b. \(\ce{Na2CO3}(aq)+\ce{H2S}(g)\rightarrow \ce{Na2S}(aq)+\ce{CO2}(g)+\ce{H2O}(l)\)
- Copper(II) sulfide is oxidized by molecular oxygen to produce gaseous sulfur trioxide and solid copper(II) oxide. The gaseous product then reacts with liquid water to produce liquid hydrogen sulfate as the only product. Write the two equations which represent these reactions.
- Write balanced chemical equations for the reactions used to prepare each of the following compounds from the given starting material(s). In some cases, additional reactants may be required.
- solid ammonium nitrate from gaseous molecular nitrogen via a two-step process (first reduce the nitrogen to ammonia, then neutralize the ammonia with an appropriate acid)
- gaseous hydrogen bromide from liquid molecular bromine via a one-step redox reaction
- gaseous H2S from solid Zn and S via a two-step process (first a redox reaction between the starting materials, then reaction of the product with a strong acid)
- Answer
- a. step 1: \(\ce{N2}(g)+\ce{3H2}(g)\rightarrow \ce{2NH3}(g)\), step 2: \(\ce{NH3}(g)+\ce{HNO3}(aq)\rightarrow \ce{NH4NO3}(aq)\rightarrow \ce{NH4NO3}(s)\ce{(after\: drying)}\); b. \(\ce{H2}(g)+\ce{Br2}(l)\rightarrow \ce{2HBr}(g)\); c. \(\ce{Zn}(s)+\ce{S}(s)\rightarrow \ce{ZnS}(s)\) and \(\ce{ZnS}(s)+\ce{2HCl}(aq)\rightarrow \ce{ZnCl2}(aq)+\ce{H2S}(g)\)
- Calcium cyclamate Ca(C6H11NHSO3)2 is an artificial sweetener used in many countries around the world but is banned in the United States. It can be purified industrially by converting it to the barium salt through reaction of the acid C6H11NHSO3H with barium carbonate, treatment with sulfuric acid (barium sulfate is very insoluble), and then neutralization with calcium hydroxide. Write the balanced equations for these reactions.
- Complete and balance each of the following half-reactions (steps 2–5 in half-reaction method):
- \(\ce{Sn^4+}(aq)\rightarrow \ce{Sn^2+}(aq)\)
- \(\ce{[Ag(NH3)2]+}(aq)\rightarrow \ce{Ag}(s)+\ce{NH3}(aq)\)
- \(\ce{Hg2Cl2}(s)\rightarrow \ce{Hg}(l)+\ce{Cl-}(aq)\)
- \(\ce{H2O}(l)\rightarrow \ce{O2}(g)\ce{\:(in\: acidic\: solution)}\)
- \(\ce{IO3-}(aq)\rightarrow \ce{I2}(s)\)
- \(\ce{SO3^2-}(aq)\rightarrow \ce{SO4^2-}(aq)\ce{\:(in\: acidic\: solution)}\)
- \(\ce{MnO4-}(aq)\rightarrow \ce{Mn^2+}(aq)\ce{\:(in\: acidic\: solution)}\)
- \(\ce{Cl-}(aq)\rightarrow \ce{ClO3-}(aq)\ce{\:(in\: basic\: solution)}\)
- Answer
- a.\(\ce{Sn^4+}(aq)+\ce{2e-}\rightarrow \ce{Sn^2+}(aq)\); b. \(\ce{[Ag(NH3)2]+}(aq)+ \ce{e-} \rightarrow \ce{Ag}(s)+\ce{2NH3}(aq)\); c. \(\ce{Hg2Cl2}(s)+ \ce{2e-} \rightarrow \ce{2Hg}(l)+\ce{2Cl-}(aq)\) ;d. \(\ce{2H2O}(l)\rightarrow \ce{O2}(g)+\ce{4H+}(aq)+\ce{4e-}\); e. \(\ce{6H2O}(l)+\ce{2IO3-}(aq)+\ce{10e-}\rightarrow \ce{I2}(s)+\ce{12OH-}(aq)\); f. \(\ce{H2O}(l)+\ce{SO3^2-}(aq)\rightarrow \ce{SO4^2-}(aq)+\ce{2H+}(aq)+\ce{2e-}\); g. \(\ce{8H+}(aq)+\ce{MnO4-}(aq)+\ce{5e-}\rightarrow \ce{Mn^2+}(aq)+\ce{4H2O}(l)\); h. \(\ce{Cl-}(aq)+\ce{6OH-}(aq)\rightarrow \ce{ClO3-}(aq)+\ce{3H2O}(l)+\ce{6e-}\)
- Complete and balance each of the following half-reactions (steps 2–5 in half-reaction method):
- \(\ce{Cr^2+}(aq)\rightarrow \ce{Cr^3+}(aq)\)
- \(\ce{Hg}(l)+\ce{Br-}(aq)\rightarrow \ce{HgBr4^2-}(aq)\)
- \(\ce{ZnS}(s)\rightarrow \ce{Zn}(s)+\ce{S^2-}(aq)\)
- \(\ce{H2}(g)\rightarrow \ce{H2O}(l)\ce{\:(in\: basic\: solution)}\)
- \(\ce{H2}(g)\rightarrow \ce{H3O+}(aq)\ce{\:(in\: acidic\: solution)}\)
- \(\ce{NO3-}(aq)\rightarrow \ce{HNO2}(aq)\ce{\:(in\: acidic\: solution)}\)
- \(\ce{MnO2}(s)\rightarrow \ce{MnO4-}(aq)\ce{\:(in\: basic\: solution)}\)
- \(\ce{Cl-}(aq)\rightarrow \ce{ClO3-}(aq)\ce{\:(in\: acidic\: solution)}\)
- Balance each of the following equations according to the half-reaction method:
- \(\ce{Sn^2+}(aq)+\ce{Cu^2+}(aq)\rightarrow \ce{Sn^4+}(aq)+\ce{Cu+}(aq)\)
- \(\ce{H2S}(g)+\ce{Hg2^2+}(aq)\rightarrow \ce{Hg}(l)+\ce{S}(s)\ce{\:(in\: acid)}\)
- \(\ce{CN-}(aq)+\ce{ClO2}(aq)\rightarrow \ce{CNO-}(aq)+\ce{Cl-}(aq)\ce{\:(in\: acid)}\)
- \(\ce{Fe^2+}(aq)+\ce{Ce^4+}(aq)\rightarrow \ce{Fe^3+}(aq)+\ce{Ce^3+}(aq)\)
- \(\ce{HBrO}(aq)\rightarrow \ce{Br-}(aq)+\ce{O2}(g)\ce{\:(in\: acid)}\)
- Answer
-
a. \(\ce{Sn^2+}(aq)+\ce{2Cu^2+}(aq)\rightarrow \ce{Sn^4+}(aq)+\ce{2Cu+}(aq)\);
b. \(\ce{H2S}(g)+\ce{Hg2^2+}(aq)+\ce{2H2O}(l)\rightarrow \ce{2Hg}(l)+\ce{S}(s)+\ce{2H3O+}(aq)\);
c. \(\ce{5CN-}(aq)+\ce{2ClO2}(aq)+\ce{3H2O}(l)\rightarrow \ce{5CNO-}(aq)+\ce{2Cl-}(aq)+\ce{2H3O+}(aq)\);
d. \(\ce{Fe^2+}(aq)+\ce{Ce^4+}(aq)\rightarrow \ce{Fe^3+}(aq)+\ce{Ce^3+}(aq)\);
e. \(\ce{2HBrO}(aq)+\ce{2H2O}(l)\rightarrow \ce{2H3O+}(aq)+\ce{2Br-}(aq)+\ce{O2}(g)\)
- Balance each of the following equations according to the half-reaction method:
- \(\ce{Zn}(s)+\ce{NO3-}(aq)\rightarrow \ce{Zn^2+}(aq)+\ce{N2}(g)\ce{\:(in\: acid)}\)
- \(\ce{Zn}(s)+\ce{NO3-}(aq)\rightarrow \ce{Zn^2+}(aq)+\ce{NH3}(aq)\ce{\:(in\: base)}\)
- \(\ce{CuS}(s)+\ce{NO3-}(aq)\rightarrow \ce{Cu^2+}(aq)+\ce{S}(s)+\ce{NO}(g)\ce{\:(in\: acid)}\)
- \(\ce{NH3}(aq)+\ce{O2}(g)\rightarrow \ce{NO2}(g)\ce{\:(gas\: phase)}\)
- \(\ce{Cl2}(g)+\ce{OH-}(aq)\rightarrow \ce{Cl-}(aq)+\ce{ClO3-}(aq)\ce{\:(in\: base)}\)
- \(\ce{H2O2}(aq)+\ce{MnO4-}(aq)\rightarrow \ce{Mn^2+}(aq)+\ce{O2}(g)\ce{\:(in\: acid)}\)
- \(\ce{NO2}(g)\rightarrow \ce{NO3-}(aq)+\ce{NO2-}(aq)\ce{\:(in\: base)}\)
- \(\ce{Fe^3+}(aq)+\ce{I-}(aq)\rightarrow \ce{Fe^2+}(aq)+\ce{I2}(aq)\)
- Balance each of the following equations according to the half-reaction method:
- \(\ce{MnO4-}(aq)+\ce{NO2-}(aq)\rightarrow \ce{MnO2}(s)+\ce{NO3-}(aq)\ce{\:(in\: base)}\)
- \(\ce{MnO4^2-}(aq)\rightarrow \ce{MnO4-}(aq)+\ce{MnO2}(s)\ce{\:(in\: base)}\)
- \(\ce{Br2}(l)+\ce{SO2}(g)\rightarrow \ce{Br-}(aq)+\ce{SO4^2-}(aq)\ce{\:(in\: acid)}\)
- Answer
-
a. \(\ce{2MnO4-}(aq)+\ce{3NO2-}(aq)+\ce{H2O}(l)\rightarrow \ce{2MnO2}(s)+\ce{3NO3-}(aq)+\ce{2OH-}(aq)\);
b. \(\ce{3MnO4^2-}(aq)+\ce{2H2O}(l)\rightarrow \ce{2MnO4-}(aq)+\ce{4OH-}(aq)+\ce{MnO2}(s)\ce{\:(in\: base)}\);
c. \(\ce{Br2}(l)+\ce{SO2}(g)+\ce{2H2O}(l)\rightarrow \ce{4H+}(aq)+\ce{2Br-}(aq)+\ce{SO4^2-}(aq)\)

