2.4: Geometries of Coordination Complexes
- Page ID
- 366301
Coordination compounds adopt geometries around the central metal ion. These geometries can be predicted by valence bond theory. The most common structures of the complexes in coordination compounds are octahedral, tetrahedral, and square planar (Figure \(\PageIndex{1}\)). For transition metal complexes, the coordination number determines the geometry around the central metal ion. Table \(\PageIndex{1}\) compares coordination numbers to the molecular geometry:
| Coordination Number | Molecular Geometry | Example |
|---|---|---|
| 2 | linear | [Ag(NH3)2]+ |
| 3 | trigonal planar | [Cu(CN)3]2− |
| 4 | tetrahedral(d0 or d10), low oxidation states for M | [Ni(CO)4] |
| 4 | square planar (d8) | [NiCl4]2− |
| 5 | trigonal bipyramidal | [CoCl5]2− |
| 5 | square pyramidal | [VO(CN)4]2− |
| 6 | octahedral | [CoCl6]3− |
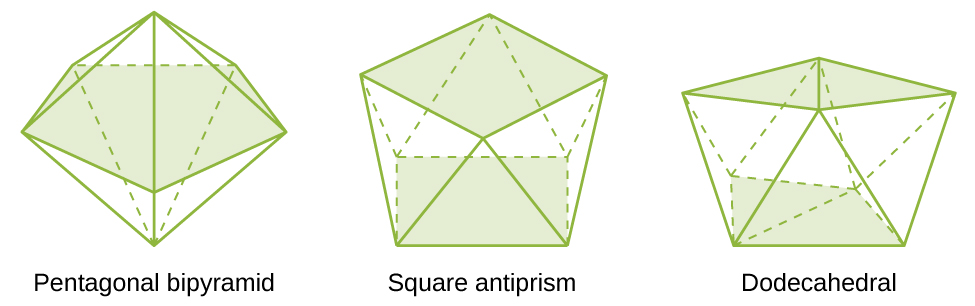
| 7 | pentagonal bipyramid | [ZrF7]3− |
| 8 | square antiprism | [ReF8]2− |
| 8 | dodecahedron | [Mo(CN)8]4− |
| 9 and above | more complicated structures | [ReH9]2− |
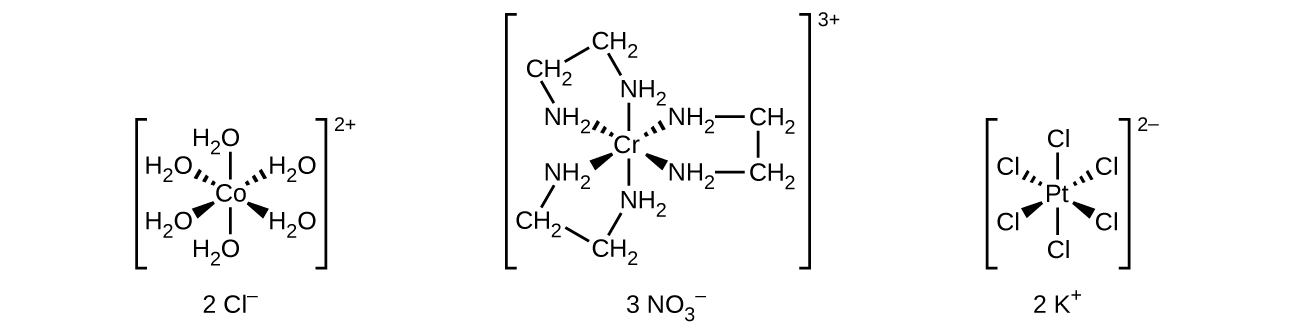
Unlike main group atoms in which both the bonding and nonbonding electrons determine the molecular shape, the nonbonding d-electrons do not change the arrangement of the ligands. Octahedral complexes have a coordination number of six, and the six donor atoms are arranged at the corners of an octahedron around the central metal ion. Examples are shown in Figure \(\PageIndex{2}\). The chloride and nitrate anions in [Co(H2O)6]Cl2 and [Cr(en)3](NO3)3, and the potassium cations in K2[PtCl6], are outside the brackets and are not bonded to the metal ion.
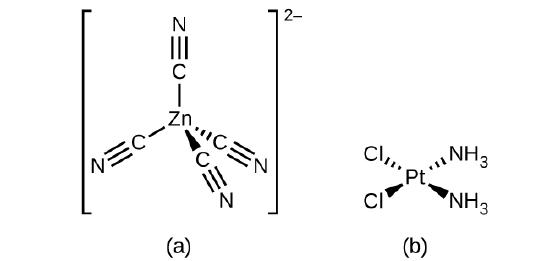
For transition metals with a coordination number of four, two different geometries are possible: tetrahedral or square planar. Unlike main group elements, where these geometries can be predicted from VSEPR theory, a more detailed discussion of transition metal orbitals (discussed in the section on Crystal Field Theory) is required to predict which complexes will be tetrahedral and which will be square planar. In tetrahedral complexes such as [Zn(CN)4]2− (Figure \(\PageIndex{3}\)), each of the ligand pairs forms an angle of 109.5°. In square planar complexes, such as [Pt(NH3)2Cl2], each ligand has two other ligands at 90° angles (called the cis positions) and one additional ligand at an 180° angle, in the trans position.
What are the geometries around each of the following complex ions?
a. [Ni(H2O)6]Cl2
b. K3[Ag(S2O3)2]
c. [Co(NH3)4Cl2]NO3
- Answer
-
a. The coordination number is 6, so the geometry is octahedral.
b. The coordination number is 2, so the geometry is linear.
c. The coordination number is 6, so the geometry is octahedral.

