3: Reactions in Aqueous Solution
- Page ID
- 189384
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)INTRODUCTION
In this experiment, we will study a number of reactions in aqueous solution. Unlike most of our experiments, you must rely heavily on qualitative observations rather than quantitative measurements. When you record an observation, it should be clear to anyone reading your notebook what you actually saw. Simply noting that a precipitate (ppt) formed is not sufficient. Adjectives describing color or consistency are often helpful; for example, “brown, powdery solid formed” or “milky, white gel formed.” Amounts and timing are also informative. Sometimes, the reaction only forms a small amount of product or it takes a few minutes to reach completion. There’s a lot to say about these “simple” reactions!
The observations that you make on Day 1 of this experiment will form the basis for the qualitative analysis on Day 2, where you will identify which cations are present in an unknown mixture.
OBJECTIVES
- To observe reactions involving some common ions and ionic compounds.
- To write the net ionic equation for each reaction observed.
- To use your observations from Day 1 of the experiment to analyze an unknown mixture for the presence of specific cations.
PROCEDURE, DAY 1: Observations of Reactions
Today, you will perform a series of tests. For each test, you must observe whether the combination of solutions results in a visible chemical change. Record your qualitative observations for each test. NOTE: When you want to observe the reaction between two solutions, you must make sure to mix them thoroughly. Usually, it is sufficient to shake the test tube you are using but you may also stir with a glass rod (just make sure it is clean to prevent contamination). Also, remember to think about the result that you are observing. Does it match your expectations based on the solubility rules?
Part 1: Precipitation Reactions
You will make sixteen different mixtures, based on the combinations suggested by the table on the following page. For each mixture, combine 2 mL of the appropriate 0.1 M metal nitrate with 2 mL of the appropriate 0.1 M sodium salt (or ammonia). Remember to thoroughly mix each combination and carefully observe the result. If a precipitate forms, note the color and consistency, and anything else of interest. If no visible change occurs, make note that no reaction occurred, or “no rxn.” Remember to consider the solubility rules. If a result does not meet your expectations, you should repeat the mixture to make sure that you performed the test correctly.
When you copy the following table into your notebook, remember to leave enough space in each box for a complete observation.
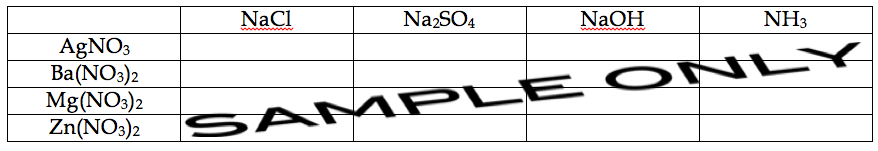
SAVE the precipitates that formed from the mixtures containing NaOH (the third column of the table). You will need these for Part 2. You may discard the other mixtures once you have recorded your observations.
Part 2: Reactions of precipitates with acid and base
Each of the precipitates that formed from mixing a metal cation with NaOH is a metal hydroxide (or oxide). You will now test how these species react with both acids and excess base. Remember to consider what you expect to happen and compare that with your actual observations. Be certain that the results of the tests are accurate and not just the result of poor technique.
- Start by taking the first sample (e.g. AgNO3 + NaOH) that you saved from Part 1 and divide it into three equal portions (you may have to stir up the precipitate if it settled).
- To the first portion, add enough 2 M HNO3 until the mixture completely reacts or you are sure that no reaction is going to occur.
- To the second portion, add enough 2 M HCl until the mixture completely reacts or you are sure that no reaction is going to occur.
- To the third portion, add enough 2 M NaOH until the mixture completely reacts or you are sure that no reaction is going to occur.
- Repeat this process with the remaining samples from Part 1.
Clearly record your observations for each test in a table similar to the one for Part 1. Note whether the hydroxide (or oxide) dissolves, changes color, remains unchanged, etc. SAVE these samples until you have decided which one that you need for Part 3.
Part 3: Reactions of an amphoteric metal hydroxide
From Part 2, you should have noticed that there was one metal hydroxide that dissolves when mixed with either acid or base. Any species that reacts with both acid and base is termed amphoteric. Decide which metal hydroxide is amphoteric and use the portion from Part 2 that contains an excess of base for this analysis.
- Pour the contents of this tube from Part 2 into a large test tube (to allow for easier mixing.)
- Test the contents with a piece of blue litmus paper. The paper should remain blue, indicating that the solution is basic. Record your observation.
- Slowly add 2 M HNO3 dropwise, mixing after each drop. Record your observations throughout this process in your notebook. Continue adding HNO3 until the solution is acidic (it should turn blue litmus paper red).
Part 4: Formation of zinc ferrocyanide
Add 2 mL of K4Fe(CN)6 (potassium ferrocyanide) solution to 2 mL of Zn(NO3)2 solution. Record your observations.
DATA ANALYSIS (Day 1)
Review your observations. Write a net ionic equation in your notebook for each combination where a reaction was observed. Refer to the lecture handouts for details. You may have noticed that some experimental observations do not match results predicted from the solubility rules. For these cases, make a note indicating the difference.
Note that when Ag+ reacts with hydroxide ions, Ag2O is the product, and not AgOH.
For Part 3, you will need a little extra detail. For certain metal ions, the species present will depend on the amount of hydroxide ions available in solution. A generic divalent metal cation will be represented here as M2+. When there is a 1:2 mole ratio of cation to hydroxide, the reaction proceeds as a normal precipitation:
M2+(aq) + 2 OH¯(aq) → M(OH)2(s)
However, if this species is amphoteric and more hydroxide ions are added, the solid can undergo a second reaction to form a soluble polyatomic ion:
M(OH)2(s) + 2 OH¯(aq) → M(OH)42¯(aq)
Use this information to write the net ionic equations for the reactions that occurred when you slowly added acid to the sample in Part 3.
DISCUSSION, DAY 2: Qualitative Analysis Through Selective Precipitation
Qualitative analysis of an ionic solution is a procedure for discovering the identity of the ions present in the mixture (rather than quantitative information about their amounts). The procedure used to separate and identify several metal cations from a single solution consists of selective precipitation.
The behavior of the four cations studied previously forms the basis for a test to detect the presence of these ions in solution. Consider a mixture, containing any or all of the cations Ag+, Ba2+, Mg2+, and Zn2+. If a HCl solution is added to this mixture and a white precipitate forms, then this confirms the presence of Ag+ (since it is the only one of these cations that forms a precipitate with chloride ions). Likewise, other specific anions can be used to selectively precipitate the other cations. The following flow chart shows the complete method you will use.
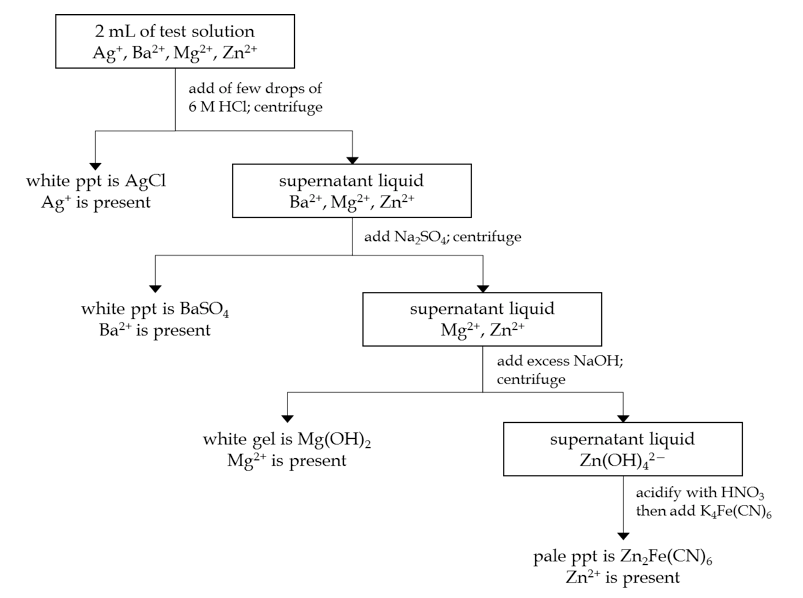
PROCEDURE DAY2: Qualitative Analysis Through Selective Precipitation
The flow chart on the previous page provides an overview, with a more detailed description of the procedure outlined below. For practice, you will first perform the entire procedure with the ALL ION solution, so that you can see the results of a positive test for each of the four cations. After that, you will be assigned an UNKNOWN sample that contains two of the four cations. Follow the procedure again with this sample and determine which cations are present.
Prepare a table in your lab notebook for both the ALL ION and UNKNOWN solutions where you will record the qualitative observations (not just a simple yes/no) for each test and your results.
Please make sure you know how to operate the centrifuge before use. Ask your nearby labmates if they also have a sample to run before you start the machine. If no precipitate forms at a particular step, there is no need to transfer the solution to a new test tube. If your test tube contains too much solution, divide it in half.
1. TEST FOR Ag+: Pour 2 mL of sample into a small test tube. Add 6 M HCl solution dropwise as long as precipitate continues to form (look carefully). Centrifuge if necessary. Pour the supernatant liquid into another small test tube.
2. TEST FOR Ba2+: To the supernatant from above, add 0.1 M Na2SO4 solution dropwise as long as precipitate continues to form (look carefully). Centrifuge if necessary. Pour the supernatant liquid into another small test tube.
3. TEST FOR Mg2+: To the supernatant from above, add excess 2 M NaOH solution (add an amount equal to roughly half of the volume of the supernatant). Centrifuge if necessary. Pour the supernatant liquid into another small test tube.
4. TEST FOR Zn2+: To the supernatant from above, add 2 M HNO3 solution until the mixture is acidic (turns blue litmus paper red). Then add several drops of K4Fe(CN)6 solution.
Your conclusion for this experiment should summarize the results of the analysis of your unknown solution (don’t forget to state its code). Which cations are present and which are not present?

