1: Measurement and Uncertainty
- Page ID
- 189382
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)INTRODUCTION
Measurements are almost never exact (unless you are counting something, like the number of students in the room). There is always some level of uncertainty in the measurements that we make in a laboratory, which will affect the precision and accuracy of our results. Precision refers to how close the individual measurements are to one another. Accuracy describes how close your average result is to the “true” or “accepted” value.
We will be using burets frequently throughout the semester, so it would be wise to test our technique with this common, but precise, instrument. To get a baseline reading of your skills, you will first calculate the density of distilled water based on some simple measurements and determine the uncertainty in your final value and its accuracy.
After you have achieved reasonable results with distilled water, you will repeat the process with an aqueous solution of sodium chloride. The density of this solution will remain unknown to you until the next lab session, so you will not be able to determine your accuracy but you will be able to determine your precision.
OBJECTIVES
1. To familiarize yourself with laboratory equipment.
2. To analyze the precision and accuracy of your measurements.
3. To calculate the density of pure water and of an aqueous solution.
PROCEDURE
NOTE: When using a buret, you must always condition it before adding the solution that you are measuring. First, check that the removable tip is secure and not loose. Next, close the stopcock and add a few (3-5) milliliters of the solution to the buret. Tip and roll the buret so that the solution coats the sides of the interior. Open the stopcock to let the solution pour out as waste. Repeat these steps for a total of three rinses. After that, your buret is ready to use. If you need your buret for another solution later, you must condition it again with the new solution. Never use soap and water to wash your buret unless it is very dirty; soap can linger through many rinses and eventually contaminate the solution you are trying to measure.
PART 1
1. Record the temperature in the room.
2. Obtain a 100-mL beaker and record its mass.
3. Add about 20 mL of distilled water to the beaker using a buret. For these measurements, you will need to make an initial buret reading before dispensing any water, followed by a final buret reading. Each reading should be estimated to the nearest hundredth of a mL. The volume of water dispensed is the difference between the two readings. Do not “aim” to get “exactly” 20 mL; this is a waste of time and will only add bias to your measurements.
4. Record the mass of the beaker with the water.
5. DO NOT empty your beaker. Repeat Steps 3 and 4 two times, using the same buret and beaker, so that you know the volume and mass of three separate samples.
6. Complete the density calculations for this part (see below) before moving on to Part 2. Show your results to your instructor to be cleared to continue.
PART 2
1. Empty and dry your 100-mL beaker or get a new one and record its mass. Every beaker is different!
2. Ask your instructor to assign you an unknown sodium chloride solution and record its ID in your notebook. Make sure you condition your buret with the new solution you are measuring. Even water can be a contaminant!
3. Add about 20 mL of sodium chloride solution to the beaker using your buret. As before, you will need an initial buret reading, followed by a final buret reading. Each reading should be estimated to the nearest hundredth of a milliliter. Again, do not “aim” to get “exactly” 20 mL.
4. Record the mass of the beaker with the solution.
5. DO NOT empty your beaker. Repeat Steps 3 and 4 two times, using the same buret and beaker, so that you know the volume and mass of three separate samples.
CALCULATIONS
1. Calculate the density of distilled water using the three trials from Part 1. Calculate the average value from your trials, the average deviation, and the uncertainty. Based on the table on the following page, calculate the percent error in your result. Show your reported value with the uncertainty and percent error to your instructor before moving on to Part 2.
2. Calculate the density of your unknown solution using the three trials from Part 2. Calculate the average value from your trials, the average deviation, and the uncertainty. You will be told the true density of this solution in the next lab period, so you cannot calculate the percent error right now.
Suggested Data Tables for Your Notebook:
Remember to neatly organize your data as you record them. A suggested layout for the data from Part 1 is given on the following page. If you write out these tables ahead of time, make sure to leave enough space in case you make any mistakes and need to correct them. Note that room was made for a fourth sample just in case one of your trials went horribly awry! DO NOT record your data directly on this sample – you have a notebook for that!
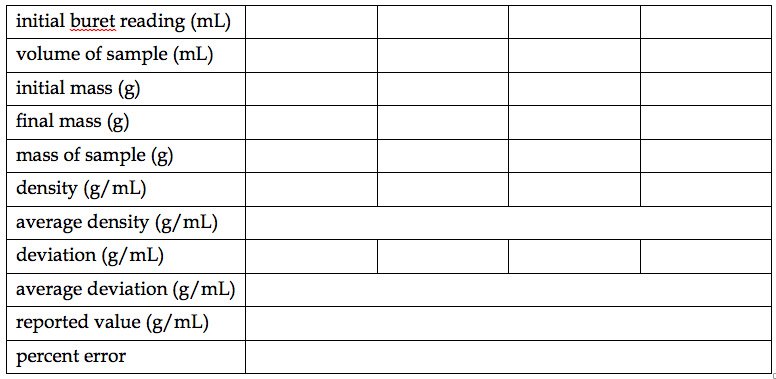
The conclusion in your notebook should include a summary of your final results (i.e. average density with uncertainty, the accepted density, and percent error) and a written statement concerning the quality of those results, along with any other notable observations from the experiment.
Density of pure water as a function of temperature
|
Temperature |
Density of pure water (g/mL) |
|
15°C |
0.9991 |
|
16°C |
0.9989 |
|
17°C |
0.9988 |
|
18°C |
0.9986 |
|
19°C |
0.9984 |
|
20°C |
0.9982 |
|
21°C |
0.9980 |
|
22°C |
0.9978 |
|
23°C |
0.9975 |
|
24°C |
0.9973 |
|
25°C |
0.9970 |

