2.4.5: Calculating Reaction Yields (Problems)
- Page ID
- 210649
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)PROBLEM \(\PageIndex{1}\)
The following quantities are placed in a container: 1.5 × 1024 molecules of diatomic hydrogen, 1.0 mol of sulfur, and 88.0 g of diatomic oxygen.
- What is the total mass in grams for the collection of all three elements?
- What is the total number of moles of atoms for the three elements?
- If the mixture of the three elements formed a compound with molecules that contain two hydrogen atoms, one sulfur atom, and four oxygen atoms, which substance is consumed first?
- How many atoms or molecules of each remaining element would remain unreacted in the change described above ?
- Answer a
-
4.98 g H2, 32 g S; 124.98 g total
- Answer b
-
2.49 mol H2, 2.75 mol O2; 6.24 mol total
- Answer c
-
S is the limiting reactant (what would be consumed first)
- Answer d
-
8.978 × 1023 molecules of H2 and 4.6 × 1023 molecules of O2 remain
- Click here to see a video of the solution
PROBLEM \(\PageIndex{2}\)
What is the limiting reactant in a reaction that produces sodium chloride from 8 g of sodium and 8 g of diatomic chlorine?
- Answer
-
The limiting reactant is Cl2
PROBLEM \(\PageIndex{3}\)
Which of the postulates of Dalton's atomic theory explains why we can calculate a theoretical yield for a chemical reaction?
- Answer
-
Postulate 4 (A compound consists of atoms of two or more elements combined in a small, whole-number ratio. In a given compound, the numbers of atoms of each of its elements are always present in the same ratio).
PROBLEM \(\PageIndex{4}\)
A student isolated 25 g of a compound following a procedure that would theoretically yield 81 g. What was his percent yield?
- Answer
-
\(\mathrm{Percent\: yield = 31\%}\)
PROBLEM \(\PageIndex{5}\)
A sample of 0.53 g of carbon dioxide was obtained by heating 1.31 g of calcium carbonate. What is the percent yield for this reaction?
\[\ce{CaCO3}(s)\rightarrow \ce{CaO}(s)+\ce{CO2}(s)\]
- Answer
-
\(\mathrm{Percent\: yield = 91.9\%}\)
- Click here to see a video of the solution
PROBLEM \(\PageIndex{6}\)
Freon-12, CCl2F2, is prepared from CCl4 by reaction with HF. The other product of this reaction is HCl. Outline the steps needed to determine the percent yield of a reaction that produces 12.5 g of CCl2F2 from 32.9 g of CCl4. Freon-12 has been banned and is no longer used as a refrigerant because it catalyzes the decomposition of ozone and has a very long lifetime in the atmosphere. Determine the percent yield.
- Answer
-
\(\ce{g\: CCl4\rightarrow mol\: CCl4\rightarrow mol\: CCl2F2 \rightarrow g\: CCl2F2}\)
\(\mathrm{\:percent\: yield=48.3\%}\)
PROBLEM \(\PageIndex{7}\)
Citric acid, C6H8O7, a component of jams, jellies, and fruity soft drinks, is prepared industrially via fermentation of sucrose by the mold Aspergillus niger. The equation representing this reaction is
\[\ce{C12H22O11 + H2O + 3O2 \rightarrow 2C6H8O7 + 4H2O}\]
What mass of citric acid is produced from exactly 1 metric ton (1.000 × 103 kg) of sucrose (C12H22O11) if the yield is 92.30%?
- Answer
-
1036 kg citric acid
- Click here to see a video of the solution
PROBLEM \(\PageIndex{8}\)
Toluene, C6H5CH3, is oxidized by air under carefully controlled conditions to benzoic acid, C6H5CO2H, which is used to prepare the food preservative sodium benzoate, C6H5CO2Na. What is the percent yield of a reaction that converts 1.000 kg of toluene to 1.21 kg of benzoic acid?
\[\ce{2C6H5CH3 + 3O2 \rightarrow 2C6H5CO2H + 2H2O}\]
- Answer
-
\(\mathrm{percent\: yield=91.3\%}\)
PROBLEM \(\PageIndex{9}\)
In a laboratory experiment, the reaction of 3.0 mol of H2 with 2.0 mol of I2 produced 1.0 mol of HI. Determine the theoretical yield in grams and the percent yield for this reaction.
- Answer
-
512 g (theoretical yield); \(\mathrm{percent\: yield=25\%}\)
- Click here to see a video of the solution
PROBLEM \(\PageIndex{10}\)
What is the limiting reactant when 1.50 g of lithium and 1.50 g of nitrogen combine to form lithium nitride, a component of advanced batteries, according to the following unbalanced equation?
\[\ce{Li + N2 \rightarrow Li3N}\]
- Answer
-
\[\ce{6Li} + \ce{N2} \rightarrow \: \ce{2Li3N}\]
\[1.50g\: \ce{Li} \times \dfrac{1\: mole\: \ce{Li}}{6.94g\: \ce{Li}} \times\dfrac{2\: mole\: \ce{Li3N}}{6\:mole\: \ce{Li}} = 0.0720\: moles\: \ce{Li3N}\]
\[1.50g\: \ce{N2} \times \dfrac{1\: mole\: \ce{N2}}{28.02g\: \ce{N2}} \times\dfrac{2\: mole\: \ce{Li3N}}{1\:mole\: \ce{N2}} = 0.107\: moles\: \ce{Li3N}\]
\(\ce{Li}\) is the limiting reactant
PROBLEM \(\PageIndex{11}\)
Uranium can be isolated from its ores by dissolving it as UO2(NO3)2, then separating it as solid UO2(C2O4)·3H2O. Addition of 0.4031 g of sodium oxalate, Na2C2O4, to a solution containing 1.481 g of uranyl nitrate, UO2(NO2)2, yields 1.073 g of solid UO2(C2O4)·3H2O.
\[\ce{Na2C2O4 + UO2(NO3)2 + 3H2O ⟶ UO2(C2O4)·3H2O + 2NaNO3}\]
Determine the limiting reactant and the percent yield of this reaction.
- Answer
-
Na2C2O4 is the limiting reactant. percent yield = 86.6%
PROBLEM \(\PageIndex{12}\)
How many molecules of C2H4Cl2 can be prepared from 15 C2H4 molecules and 8 Cl2 molecules?
- Answer
-
Only 8 molecules can be formed
PROBLEM \(\PageIndex{13}\)
How many molecules of the sweetener saccharin can be prepared from 30 C atoms, 25 H atoms, 12 O atoms, 8 S atoms, and 14 N atoms?
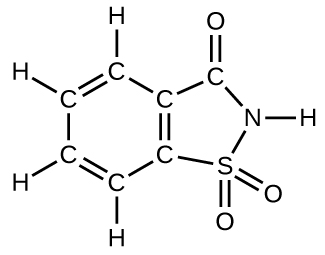
- Answer
-
Only four molecules can be made.
PROBLEM \(\PageIndex{14}\)
The phosphorus pentoxide used to produce phosphoric acid for cola soft drinks is prepared by burning phosphorus in oxygen.
- What is the limiting reactant when 0.200 mol of P4 and 0.200 mol of O2 react according to \[\ce{P4 + 5O2 \rightarrow P4O10}\]
- Calculate the percent yield if 10.0 g of P4O10 is isolated from the reaction.
- Answer a
-
O2 is the limiting reactant
- Answer b
-
\(\mathrm{percent\: yield=88\%}\)
- Click here to see a video of the solution
PROBLEM \(\PageIndex{15}\)
Would you agree to buy 1 trillion (1,000,000,000,000) gold atoms for $5? Explain why or why not. Find the current price of gold at http://money.cnn.com/data/commodities/ \(\mathrm{(1\: troy\: ounce=31.1\: g)}\)
- Answer
-
This amount cannot be weighted by ordinary balances and is worthless.
Have a video solution request?
Let your professors know here.
***Please know that you are helping future students - videos will be made in time for next term's class.
Contributors and Attributions
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
- Adelaide Clark, Oregon Institute of Technology
Feedback
Think one of the answers above is wrong? Let us know here.

