6.3: Citric Acid Cycle and Related Pathways
- Page ID
- 347438
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Source: BiochemFFA_6_2.pdf. The entire textbook is available for free from the authors at http://biochem.science.oregonstate.edu/content/biochemistry-free-and-easy
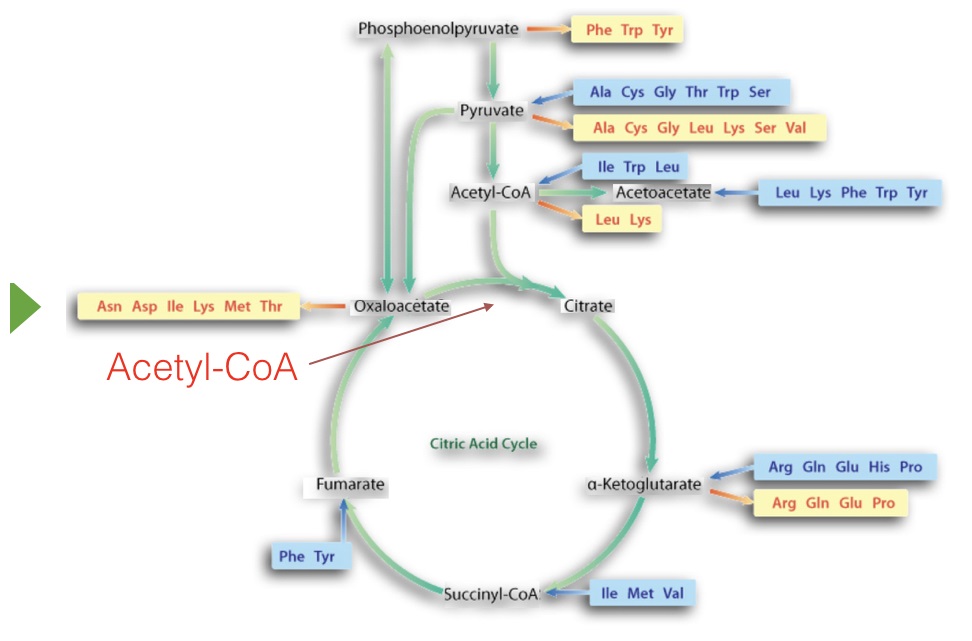
Citric acid cycle
The primary catabolic pathway in the body is the citric acid cycle because it is here that oxidation to carbon dioxide occurs for breakdown products of the cell’s major building blocks - sugars, fatty acids, and amino acids. The pathway is cyclic (Figure 6.63) and thus, doesn’t really have a starting or ending point. All of the reactions occur in mitochondria, though one enzyme is embedded in the organelle’s inner membrane. As needs change, cells may use a subset of the reactions of the cycle to produce the desired molecules via partial cycle rather than to run the entire cycle.
Acetyl-CoA
The molecule “feeding” the citric acid cycle is acetyl-CoA and it can be obtained from pyruvate (from glycolysis), from fatty acid β-oxidation, from ketone bodies, and from amino acid metabolism. Molecules from other pathways feeding into the citric acid cycle for catabolism make the citric acid cycle ‘cataplerotic’. It is worth noting that acetyl-CoA has very different fates, depending on the cell’s energy status/needs (see HERE). The description below describes oxidation (catabolism) in citric acid cycle.
Anabolically, acetyl-CoA is also very important for providing building blocks for synthesis of fatty acids, ketone bodies, amino acids and cholesterol. Other citric acid cycle intermediates are also important in amino acid metabolism (Figure 6.63), heme synthesis, electron shuttling, and shuttling of acetyl-CoA across the mitochondrial inner membrane. The ability of the citric acid cycle to supply intermediates to pathways gives rise to the term ‘anaplerotic.’ It means ‘to fill up.’ Before discussing the citric acid cycle, it is important to first describe one important enzyme complex that is a major source of acetyl-CoA for the cycle.
Figure 6.64 - E1 Subunit of Pyruvate Dehydrogenase. Wikipedia
Pyruvate decarboxylation
The pyruvate dehydrogenase enzyme is a complex of multiple copies of three subunits that catalyze the decarboxylation of pyruvate to form acetyl-CoA. The reaction mechanism requires use of five coenzymes. Pyruvate dehydrogenase is an enormous complex in mammals with a size five times greater than ribosomes.
Subunits
The three subunits are designated by E1, E2, and E3. E2 is also referred to as dihydrolipoamide acetyltransferase and E3 is more precisely called dihydrolipoyl dehydrogenase. Confusion arises with the name for E1. Some call it pyruvate dehydrogenase and others give it the name pyruvate decarboxylase. We will use pyruvate decarboxylase solely to refer to E1 and pyruvate dehydrogenase only to refer to the complex of E1, E2, and E3.
The catalytic actions of pyruvate dehydrogenase can be broken down into three steps, each taking place on one of the subunits. The steps, sequentially occurring on E1, E2, and E3, are 1) decarboxylation of pyruvate; 2) oxidation of the decarboxylated product; and 3) transfer of electrons to ultimately form NADH (Figure 6.65).
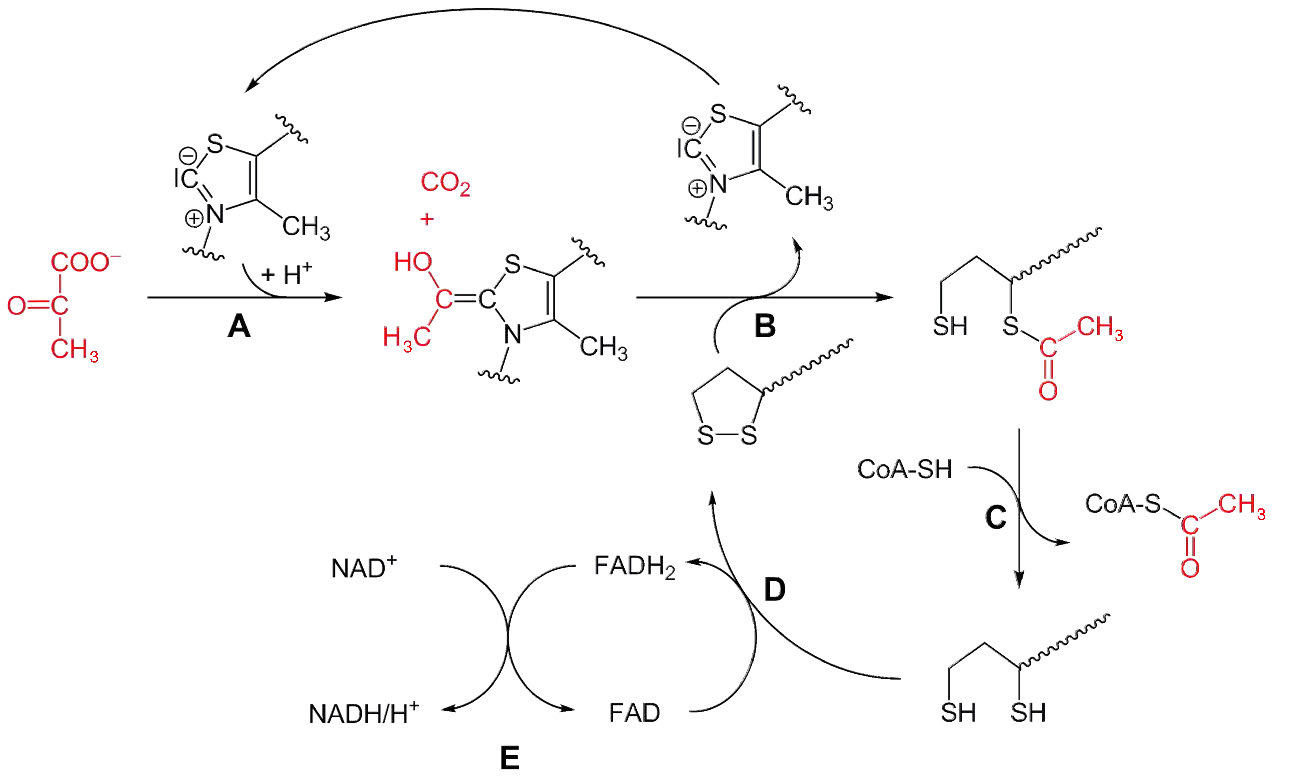
Figure 6.65 - Mechanism of action of pyruvate decarboxylation and oxidation by pyruvate dehydrogenase.
Catalysis
The catalytic process begins after binding of the pyruvate substrate with activation of the thiamine pyrophosphate coenzyme through formation of an ylide intermediate. The nucleophilic carbanion of the ylide attacks the electrophilic ketone carbon on the pyruvate, releasing carbon dioxide and creating an enol that loses a proton on the carbon to become a 1,3 dipole that includes the positively charged nitrogen of the thiamine. The reaction (step A in Figure 6.65) is a non-oxidative decarboxylation. Oxidation of the two carbon hydroxyethyl unit occurs in the transfer to the lipoamide.
Reductive acetylation
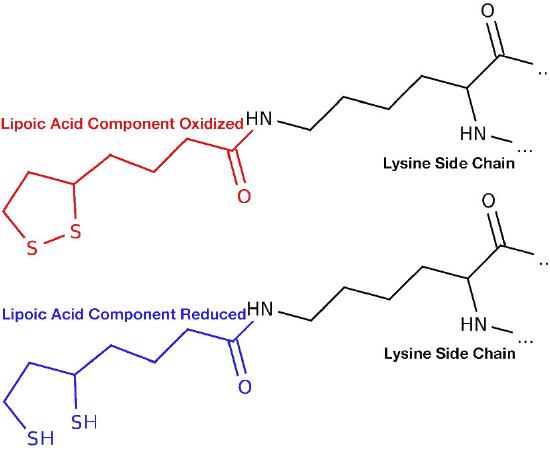
Reductive acetylation occurs next (Step B) as the 2-carbon hydroxyethyl unit is transferred to lipoamide on E2. (Lipoamide is the name for a molecule of lipoic acid covalently attached to a lysine side chain in the E2 subunit). In prokaryotes in the absence of oxygen, the hydroxyethyl group is not passed to lipoamide, but instead is released as free acetaldehyde , which can accept electrons from NADH (catalyzed by alcohol dehydrogenase) and become ethanol in the process of fermentation. In the presence of oxygen in almost all aerobic organisms, the process continues with transfer of the hydroxyethyl unit to E2 and continuation of the cycle below.
Oxidation step
Transfer of the hydroxyethyl group from E1 to the lipoamide coenzyme in E2 is an oxidation, with transfer of electrons from the hydroxyethyl group to lipoamide’s disulfide (reducing it) and formation on the lipoamide of an acetyl-thioester (oxidizing it).
The acetyl group is then transferred from lipoamide to coenzyme A in E2 (Step C in Figure 6.65), forming acetyl-CoA, which is released and leaving reduced sulfhydryls on the lipoamide. In order for the enzyme to return to its original state, the disulfide bond on lipoamide must be re-formed. This occurs with transfer of electrons from reduced lipoamide to an FAD covalently bound to E3 (Step D). This reduces FAD to FADH2.
Formation of NADH
In the last step in the process, electrons from FADH2 are transferred to external NAD+, forming NADH (Step E) and completing the overall cycle. Then enzyme can then begin another catalytic round by binding to a pyruvate.
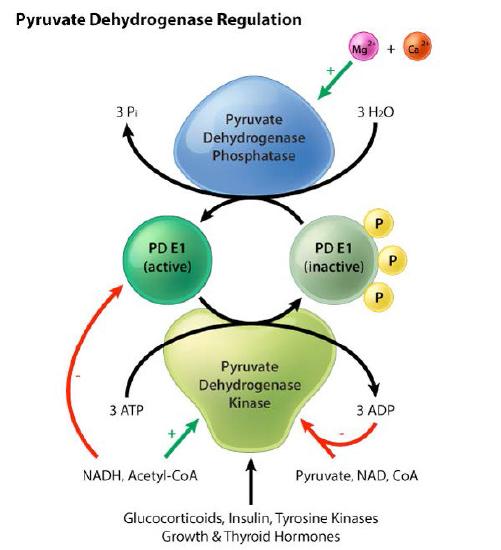
Pyruvate dehydrogenase regulation
Pyruvate deyhdrogenase is regulated both allosterically and by covalent modification - phosphorylation / dephosphorylation. Regulation of pyruvate dehydrogenase, whether by allosteric or covalent mechanisms has the same strategy. Indicators of high energy shut down the enzyme, whereas indicators of low energy stimulate it. For allosteric regulation, the high energy indicators affecting the enzyme are ATP, acetyl-CoA, NADH, and fatty acids, which inhibit it. AMP, Coenzyme A, NAD+, and calcium, on the other hand, stimulate it (Figure 6.67).
Covalent modification
Covalent modification regulation of pyruvate dehydrogenase is a bit more complicated. It occurs as a result of phosphorylation by pyruvate dehydrogenase kinase (PDK - Figure 6.67) or dephosphorylation by pyruvate dehydrogenase phosphatase (PDP).
PDK puts phosphate on any one of three serine residues on the E1 subunit, which causes pyruvate kinase to not be able to perform its first step of catalysis - the decarboxylation of pyruvate. PDP can remove those phosphates. PDK is allosterically activated in the mitochondrial matrix when NADH and acetyl-CoA concentrations rise.
Product inhibition
Thus, the products of the pyruvate dehydrogenase reaction inhibit the production of more products by favoring its phosphorylation by PDK. Pyruvate, a substrate of pyruvate dehydrogenase, inhibits PDK, so increasing concentrations of substrate activate pyruvate dehydrogenase by reducing its phosphorylation by PDK. As concentrations of NADH and acetyl-CoA fall, PDP associates with pyruvate kinase and removes the phosphate on the serine on the E1 subunit.

Low concentrations of NADH and acetyl-CoA are necessary for PDP to remain on the enzyme. When those concentrations rise, PDP dissociates and PDK gains access to the serine for phosphorylation. Insulin and calcium can also activate the PDP. This is very important in muscle tissue, since calcium is a signal for muscular contraction, which requires energy. Insulin also also activates pyruvate kinase and the glycolysis pathway to use internalized glucose. It should be noted that the cAMP phosphorylation cascade from the β-adrenergic receptor has no effect on pyruvate kinase, though the insulin cascade does, in fact, affect PDP and pyruvate kinase.

Citric acid cycle reactions
Focusing on the pathway itself (Figure 6.69), the usual point to start discussion is addition of acetyl-CoA to oxaloacetate (OAA) to form citrate.
Acetyl-CoA for the pathway can come from a variety of sources. The reaction joining it to OAA is catalyzed by citrate synthase and the ∆G°’ is fairly negative. This, in turn, helps to “pull” the malate dehydrogenase reaction preceding it in the cycle.
In the next reaction, citrate is isomerized to isocitrate by action of the enzyme called aconitase.
Isocitrate is a branch point in plants and bacteria for the glyoxylate cycle (see HERE). Oxidative decarboxylation of isocitrate by isocitrate dehydrogenase produces the first NADH and yields α-ketoglutarate.
This five carbon intermediate is a branch point for synthesis of the amino acid glutamate. In addition, glutamate can also be made easily into this intermediate in the reverse reaction. Decarboxylation of α-ketoglutarate produces succinyl-CoA and is catalyzed by α-ketoglutarate dehydrogenase.
The enzyme α-ketoglutarate dehydrogenase is structurally very similar to pyruvate dehydrogenase and employs the same five coenzymes – NAD+, FAD, CoA-SH, thiamine pyrophosphate, and lipoamide.
Regeneration of oxaloacetate
The remainder of the citric acid cycle involves conversion of the four carbon succinyl-CoA into oxaloacetate. Succinyl-CoA is a branch point for the synthesis of heme (see HERE). Succinyl-CoA is converted to succinate in a reaction catalyzed by succinyl-CoA synthetase (named for the reverse reaction) and a GTP is produced, as well – the only substrate level phosphorylation in the cycle.
The energy for the synthesis of the GTP comes from hydrolysis of the high energy thioester bond between succinate and the CoA-SH. Evidence for the high energy of a thioester bond is also evident in the citrate synthase reaction, which is also very energetically favorable. Succinate is also produced by metabolism of odd-chain fatty acids (see HERE).
Succinate Oxidation
Oxidation of succinate occurs in the next step, catalyzed by succinate dehydrogenase. This interesting enzyme both catalyzes this reaction and participates in the electron transport system, funneling electrons from the FADH2 it gains in the reaction to coenzyme Q. The product of the reaction, fumarate, gains a water across its trans double bond in the next reaction, catalyzed by fumarase to form malate.
Fumarate is also a byproduct of nucleotide metabolism and of the urea cycle. Malate is important also for transporting electrons across membranes in the malate-aspartate shuttle (see HERE) and in ferrying carbon dioxide from mesophyll cells to bundle sheath cells in C4 plants (see HERE).
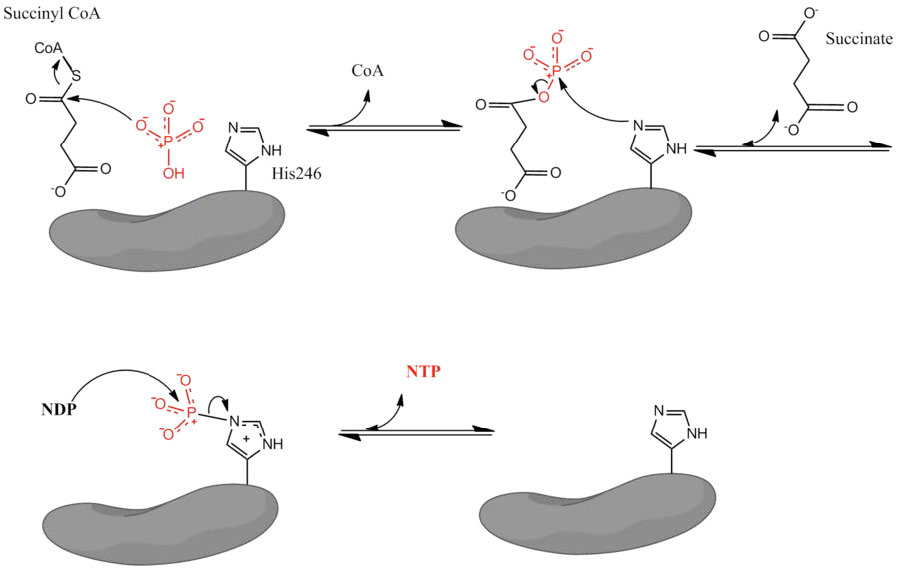

Rare oxidation
Conversion of malate to oxaloacetate by malate dehydrogenase is a rare biological oxidation that has a ∆G°’ with a positive value (29.7 kJ/mol).
The reaction is ‘pulled’ by the energetically favorable conversion of oxaloacetate to citrate in the citrate synthase reaction described above. Oxaloacetate intersects other important pathways, including amino acid metabolism (readily converted to aspartic acid), transamination (nitrogen movement) and gluconeogenesis.
It is worth noting that reversal of the citric acid cycle theoretically provides a mechanism for assimilating CO2. In fact, this reversal has been noted in both anaerobic and microaerobic bacteria, where it is called the Arnon-Buchanan cycle (Figure 6.73).
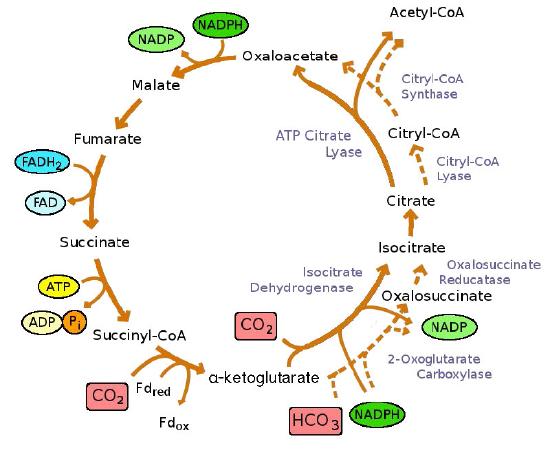
Regulation of the citric acid cycle
Allosteric regulation of the citric acid cycle is pretty straightforward. The molecules involved are all substrates/products of the pathway or molecules involved in energy transfer. Substrates/products that regulate or affect the pathway include acetyl-CoA and succinyl-CoA .
Inhibitors and activators
High energy molecular indicators, such as ATP and NADH will tend to inhibit the cycle and low energy indicators (NAD+, AMP, and ADP) will tend to activate the cycle. Pyruvate dehydrogenase, which catalyzes formation of acetyl-CoA for entry into the cycle is allosterically inhibited by its product (acetyl-CoA), as well as by NADH and ATP.
Regulated enzymes
Regulated enzymes in the cycle include citrate synthase (inhibited by NADH, ATP, and succinyl-CoA), isocitrate dehydrogenase (inhibited by ATP, activated by ADP and NAD+), and α-ketoglutarate dehydrogenase (inhibited by NADH and succinyl-CoA and activated by AMP).
Anaplerotic/cataplerotic pathway
The citric acid cycle is an important catabolic pathway oxidizing acetyl-CoA into CO2 and generating ATP, but it is also an important source of molecules needed by cells and a mechanism for extracting energy from amino acids in protein breakdown and other breakdown products. This ability of the citric acid cycle to supply molecules as needed and to absorb metabolic byproducts gives great flexibility to cells. When citric acid cycle intermediates are taken from the pathway to make other molecules, the term used to describe this is cataplerotic, whereas when molecules are added to the pathway, the process is described as anaplerotic.
Cataplerotic molecules
The citric acid cycle’s primary cataplerotic molecules include α-ketoglutarate, succinyl-CoA, and oxaloacetate. Transamination of α-ketoglutarate and oxaloacetate produces the amino acids glutamate and aspartic acid, respectively. Oxaloacetate is important for the production of glucose in gluconeogenesis.
Glutamate plays a very important role in the movement of nitrogen through cells via glutamine and other molecules and is also needed for purine synthesis. Aspartate is a precursor of other amino acids and for production of pyrimidine nucleotides. Succinyl-CoA is necessary for the synthesis of porphyrins, such as the heme groups in hemoglobin, myoglobin and cytochromes.
Citrate is an important source of acetyl-CoA for making fatty acids. When the citrate concentration is high (as when the citric acid cycle is moving slowly or is stopped), it gets shuttled across the mitochondrial membrane into the cytoplasm and broken down by the enzyme citrate lyase to oxaloacetate and acetyl-CoA. The latter is a precursor for fatty acid synthesis in the cytoplasm.
Anaplerotic molecules
Anaplerotic molecules replenishing citric acid cycle intermediates include acetyl-CoA (made in many pathways, including fatty acid oxidation, pyruvate decarboxylation, amino acid catabolism, and breakdown of ketone bodies), α-ketoglutarate (from amino acid metabolism), succinyl-CoA (from propionic acid metabolism), fumarate (from the urea cycle and purine metabolism), malate (carboxylation of PEP in plants), and oxaloacetate (many sources, including amino acid catabolism and pyruvate carboxylase action on pyruvate in gluconeogenesis)
Glyoxylate cycle
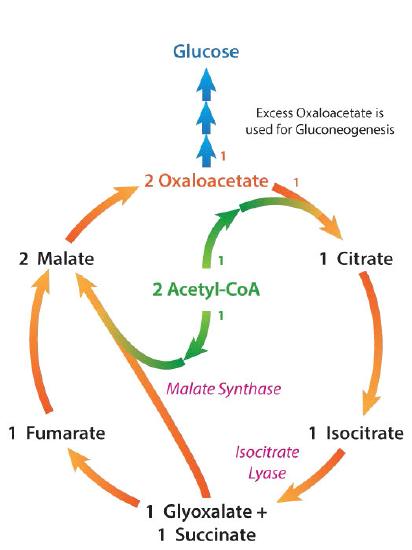

A pathway related to the citric acid cycle found only in plants and bacteria is the glyoxylate cycle (Figures 6.74 & 6.75). The glyoxylate cycle, which bypasses the decarboxylation reactions while using most of the non-decarboxylation reactions of the citric acid cycle, does not operate in animals, because they lack two enzymes necessary for it – isocitrate lyase and malate synthase. The cycle occurs in specialized plant peroxisomes called glyoxysomes. Isocitrate lyase catalyzes the conversion of isocitrate into succinate and glyoxylate. Because of this, all six carbons of the citric acid cycle survive each turn of the cycle and do not end up as carbon dioxide.
Succinate continues through the remaining reactions to produce oxaloacetate. Glyoxylate combines with another acetyl-CoA (one acetyl-CoA was used to start the cycle) to create malate (catalyzed by malate synthase). Malate can, in turn, be oxidized to oxaloacetate.
It is at this point that the glyoxylate pathway’s contrast with the citric acid cycle is apparent. After one turn of the citric acid cycle, a single oxaloacetate is produced and it balances the single one used in the first reaction of the cycle. Thus, in the citric acid cycle, there is no net production of oxaloacetate in each turn of the cycle.
Net oxaloacetate production
On the other hand, thanks to assimilation of carbons from two acetyl-CoA molecules, each turn of the glyoxylate cycle results in two oxaloacetates being produced, after starting with one. The extra oxaloacetate of the glyoxylate cycle can be used to make other molecules, including glucose in gluconeogenesis. This is particularly important for plant seed germination (Figure 6.76), since the seedling is not exposed to sunlight. With the glyoxylate cycle, seeds can make glucose from stored lipids.
Because animals do not run the glyoxylate cycle, they cannot produce glucose from acetyl-CoA in net amounts, but plants and bacteria can. As a result, plants and bacteria can turn acetyl-CoA from fat into glucose, while animals can’t. Bypassing the oxidative decarboxylations (and substrate level phosphorylation) has energy costs, but, there are also benefits. Each turn of the glyoxylate cycle produces one FADH2 and one NADH instead of the three NADHs, one FADH2, and one GTP made in each turn of the citric acid cycle.

Carbohydrate needs
Organisms that make cell walls, such as plants, fungi, and bacteria, need large quantities of carbohydrates as they grow to support the biosynthesis of the complex structural polysaccharides of the walls. These include cellulose, glucans, and chitin. Notably, each of the organisms can operate the glyoxylate cycle using acetyl-CoA from β-oxidation.
Coordination of the glyoxylate cycle and the citric acid cycle
The citric acid cycle is a major catabolic pathway producing a considerable amount of energy for cells, whereas the glyoxylate cycle’s main function is anabolic - to allow production of glucose from fatty acids in plants and bacteria. The two pathways are physically separated from each other (glyoxylate cycle in glyoxysomes / citric acid cycle in mitochondria), but nonetheless a coordinated regulation of them is important.
The enzyme that appears to provide controls for the cycle is isocitrate dehydrogenase. In plants and bacteria, the enzyme can be inactivated by phosphorylation by a kinase found only in those cells. Inactivation causes isocitrate to accumulate in the mitochondrion and when this happens, it gets shunted to the glyoxysomes, favoring the glyoxylate cycle. Removal of the phosphate from isocitrate dehydrogenase is catalyzed by an isocitrate dehydrognease-specific phosphoprotein phosphatase and restores activity to the enzyme.
When this happens, isocitrate oxidation resumes in the mitochondrion along with the rest of the citric acid cycle reactions. In bacteria, where the enzymes for both cycles are present together in the cytoplasm, accumulation of citric acid cycle intermediates and glycolysis intermediates will tend to favor the citric acid cycle by activating the phosphatase, whereas high energy conditions will tend to favor the glyoxylate cycle by inhibiting it.
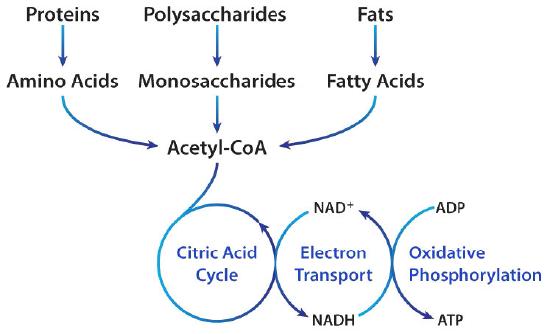
Acetyl-CoA metabolism
Acetyl-CoA is one of the most “connected” metabolites in biochemistry, appearing in fatty acid oxidation/synthesis, pyruvate oxidation, the citric acid cycle, amino acid anabolism/catabolism, ketone body metabolism, steroid/bile acid synthesis, and (by extension from fatty acid metabolism) prostaglandin synthesis . Most of these pathways will be dealt with separately. Here we will cover ketone body metabolism.
Ketone body metabolism
Ketone bodies are molecules made when the blood levels of glucose fall very low. Ketone bodies can be converted to acetyl-CoA by reversing the reaction of the pathway that makes them (Figure 6.78). Acetyl CoA, of course, can be used for ATP synthesis via the citric acid cycle. People who are very hypoglycemic (including some diabetics) will produce ketone bodies (Figure 6.79) and these are often first detected by the smell of acetone on their breath.
Overlapping pathways
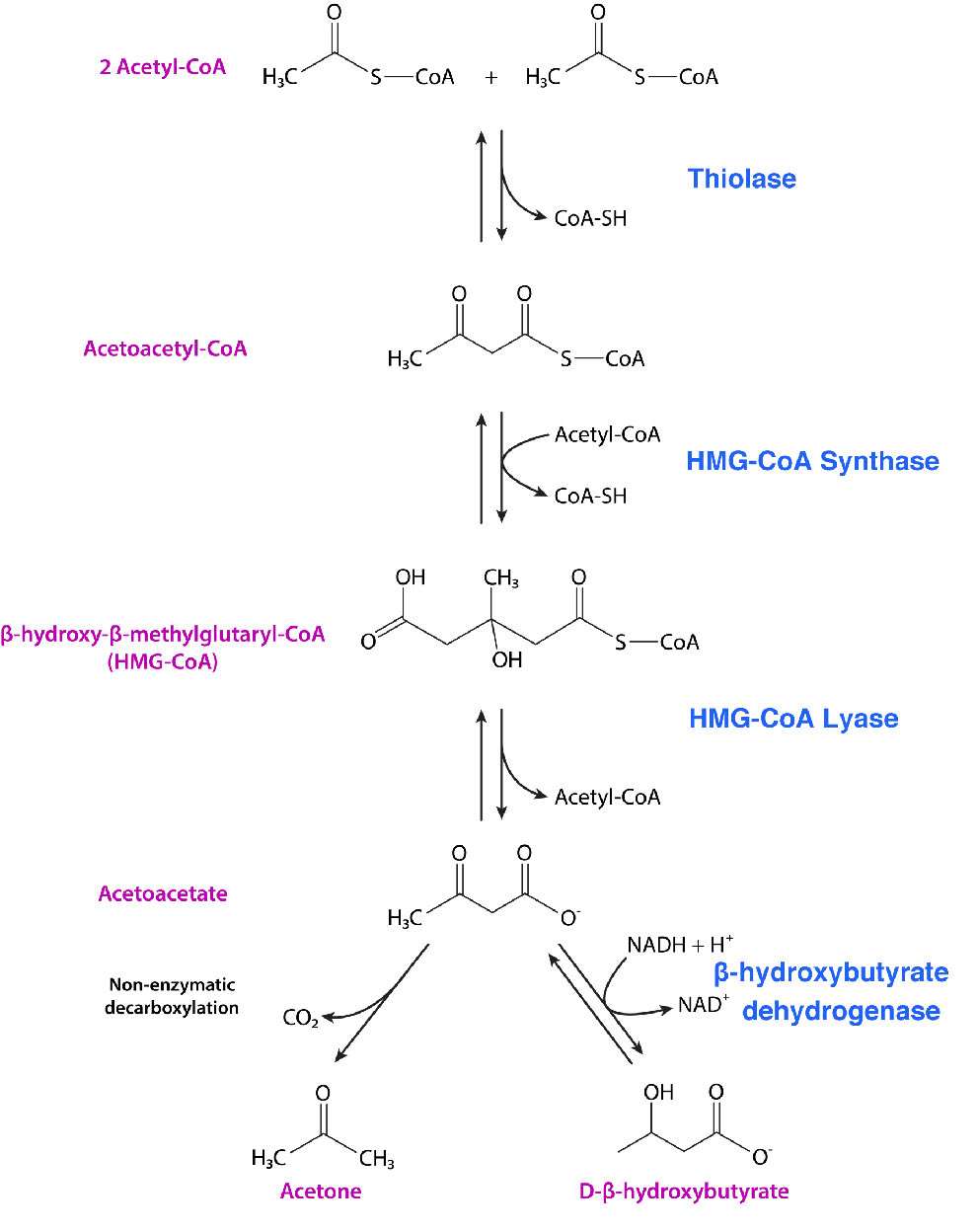
The pathways for ketone body synthesis and cholesterol biosynthesis (Figure 6.80 and see HERE) overlap at the beginning. Each of these starts by combining two acetyl-CoAs together to make acetoacetyl-CoA. Not coincidentally, that is the next to last product of β-oxidation of fatty acids with even numbers of carbons (see HERE for fatty acid oxidation). In fact, the enzyme that catalyzes the joining is the same as the one that catalyzes its breakage in fatty acid oxidation – thiolase. Thus, these pathways start by reversing the last step of the last round of fatty acid oxidation.
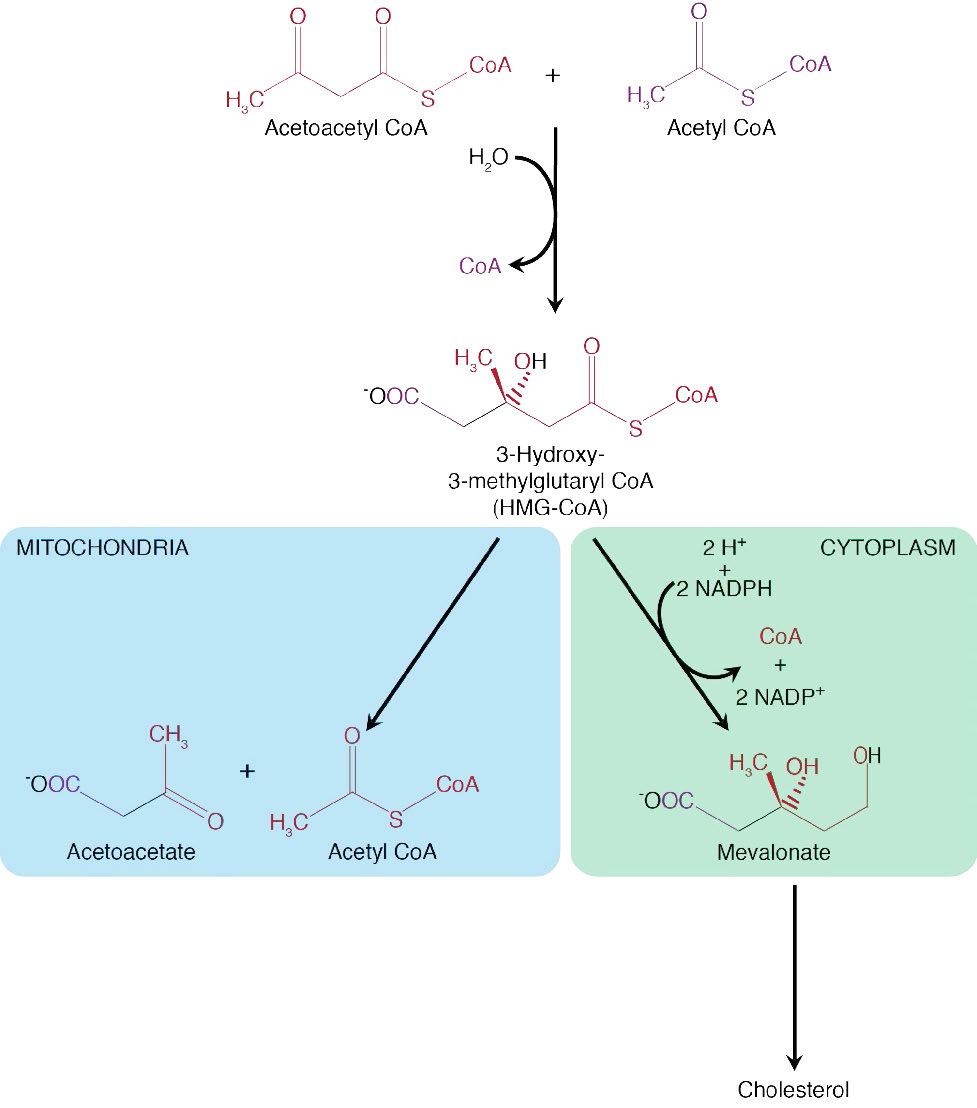
HMG-CoA formation
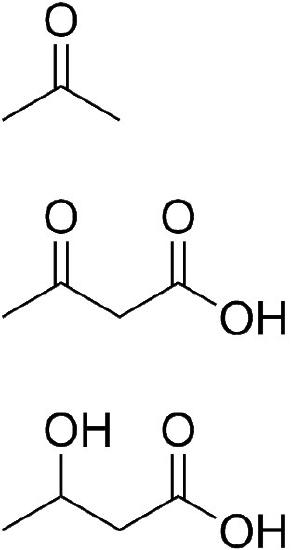
Both pathways also include addition of two more carbons to acetoacetyl-CoA from a third acetyl-CoA to form hydroxy-methyl-glutaryl-CoA, or HMG-CoA, as it is more commonly known. It is at this point that the two pathways diverge. HMG-CoA is a branch point between the two pathway and can either go on to become cholesterol or ketone bodies. In the latter pathway, HMG-CoA is broken down into acetyl-CoA and acetoacetate.
Acetoacetate is itself a ketone body and can be reduced to form another one, D-β-hydroxybutyrate (not actually a ketone, though). Alternatively, acetoacetate can be converted into acetone. This latter reaction can occur either spontaneously or via catalysis by acetoacetate decarboxylase. Acetone can be converted into pyruvate and pyruvate can be made into glucose.
D-β-hydroxybutyrate travels readily in the blood and crosses the blood-brain barrier. It can be oxidized back to acetoacetate, converted to acetoacetyl-CoA, and then broken down to two molecules of acetyl-CoA for oxidation in the citric acid cycle.
Ketosis
When a body is producing ketone bodies for its energy, this state in the body is known as ketosis. Formation of ketone bodies in the liver is critical. Normally glucose is the body’s primary energy source. It comes from the diet, from the breakdown of storage carbohydrates, such as glycogen, or from glucose synthesis (gluconeogenesis). Since the primary stores of glycogen are in muscles and liver and since gluconeogenesis occurs only in liver, kidney, and gametes, when the supply of glucose is interrupted for any reason, the liver must supply an alternate energy source.
From fatty acid breakdown
In contrast to glucose, ketone bodies can be made in animals from the breakdown of fat/fatty acids. Most cells of the body can use ketone bodies as energy sources. Ketosis may arise from fasting, a very low carbohydrate diet or, in some cases, diabetes.
Acidosis
The term acidosis refers to conditions in the body where the pH of arterial blood drops below 7.35. It is the opposite of the condition of alkalosis, where the pH of the arterial blood rises above 7.45. Normally, the pH of the blood stays in this narrow pH range. pH values of the blood lower than 6.8 or higher than 7.8 can cause irreversible damage and may be fatal. Acidosis may have roots in metabolism (metabolic acidosis) or in respiration (respiratory acidosis).
There are several causes of acidosis. In metabolic acidosis, production of excess lactic acid or failure of the kidneys to excrete acid can cause blood pH to drop. Lactic acid is produced in the body when oxygen is limiting, so anything that interferes with oxygen delivery may create conditions favoring production of excess lactic acid. These may include restrictions in the movement of blood to target tissues, resulting in hypoxia (low oxygen conditions) or decreases in blood volume. Issues with blood movement can result from heart problems, low blood pressure, or hemorrhaging.
Strenuous exercise can also result in production of lactic acid due to the inability of the blood supply to deliver oxygen as fast as tissues require it (hypovolemic shock). At the end of the exercise, though, the oxygen supply via the blood system quickly catches up.
Respiratory acidosis arises from accumulation of carbon dioxide in the blood. Causes include hypoventilation, pulmonary problems, emphysema, asthma, and severe pneumonia.

Figure 6.81 - Symptoms of acidosis


