5.4: Transport Across Membranes
- Page ID
- 347419
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Source: BiochemFFA_3_2.pdf. The entire textbook is available for free from the authors at http://biochem.science.oregonstate.edu/content/biochemistry-free-and-easy
Movement of materials across membranes
As noted earlier, it is essential for cells to be able to uptake nutrients. This function along with movement of ions and other substances is provided by proteins/protein complexes that are highly specific for the compounds they move.
Selective movement of ions by membrane proteins and the ions’ extremely low permeability across the lipid bilayer are important for helping to maintain the osmotic balance of the cell and also for providing for the most important mechanism for it to make ATP - the process of oxidative phosphorylation.
Terminology
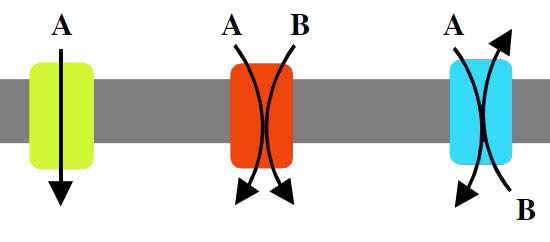
A protein involved in moving only one molecule across a membrane is called a uniport (Figure 3.25). Proteins that move two molecules in the same direction across the membrane are called symports (also called synporters, synports, or symporters). If two molecules are moved in opposite directions across the bilayer, the protein is called an antiport. Proteins involved in moving ions are called ionophores.
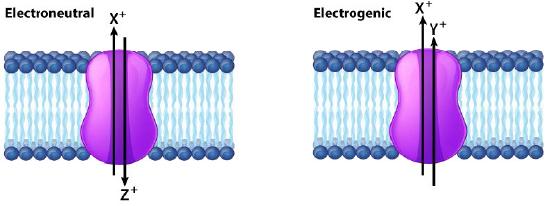
If the action of a protein in moving ions across a membrane results in a net change in charge, the protein is described as electrogenic and if there is no change in charge the protein is described as electroneutral (Figure 3.26). When the driving force for movement through the membrane protein is simply diffusion, the process is called facilitated diffusion or passive transport and when the process requires other energy input, the process is called active transport.
Channels and transporters
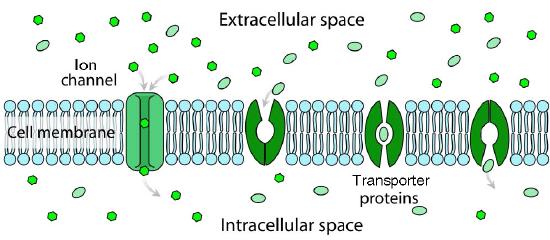
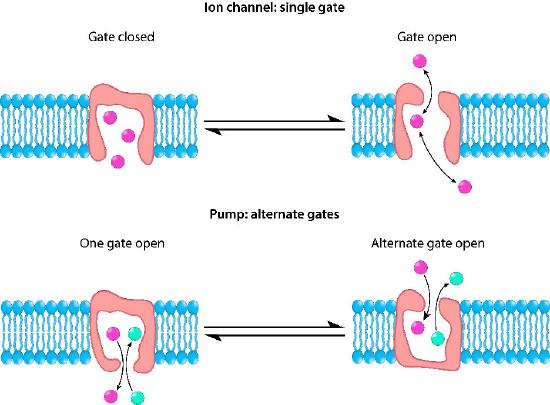
With respect to movement of materials through membrane proteins, there is a difference between channels (sometimes called pores) and transporters. Channels largely provide openings with some specificity and molecules pass through them at close to the rate of diffusion. They usually involve movement of water or ions. Examples would be the sodium or potassium channels of nerve cells. Transporters have high specificity and transfer rates that are orders of magnitude slower. Transport proteins include the sodium-potassium pump, the sodium-calcium exchanger, and lactose permease, amongst many others).
Facilitated diffusion
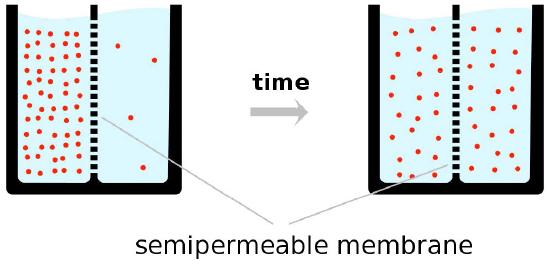
As noted, the driving force for facilitated diffusion is concentration, meaning that in facilitated diffusion, materials will only move from a higher concentration to a lower concentration and that at the end of the process, the concentration of materials on each side of a bilayer will be equal (Figure 3.28). This may work well in many cases.
For example, the blood concentration of glucose is sufficiently high that red blood cells can use facilitated diffusion as a means of acquiring glucose. Other cells, further removed from the blood supply where the glucose concentration is lower, must use active transport mechanisms because there is not a sufficient concentration of glucose to provide cells with the glucose they need.
Ion channels
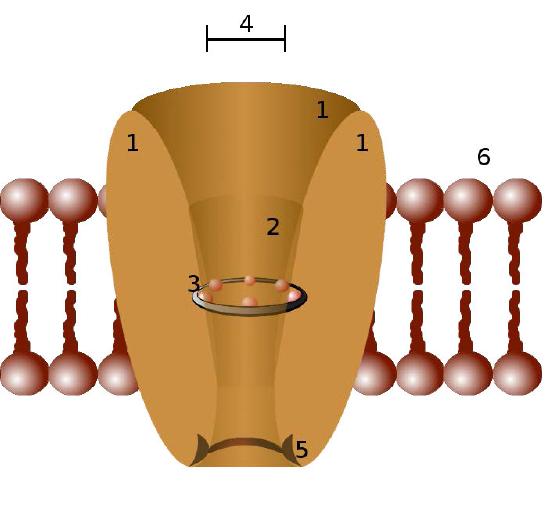
Ion channels are pore-forming membrane proteins in the membranes of all cells that regulate movement of selected ions across a membrane (Figures 3.29 & 3.30). They help to establish the resting membrane potential and to affect action potentials and other electrical signals. They are very important in the process of nerve transmission. Ion channels control the flow of ions across secretory and epithelial cells, and consequently help to regulate cell volume by affecting osmotic pressure.
Ion channels are essential features of almost all cells, functioning as selective “tunnels” that restrict movement through them to ions with specific characteristics (typically size). The size of the opening is very narrow (usually one or two atoms wide) and is able to select even against ions that are too small.
Control mechanisms
Ion channels are controlled by mechanisms that include voltage, ligands, light, temperature, and mechanical deformation (stretch activated). Ligand-gated ion channels (LGICs) are transmembrane proteins which open to selectively allow ions such as Na+, K+, Ca++, or Cl− to pass through the membrane in response to the binding of a ligand messenger.
Sound waves cause mechanical deformation of hair cells in the ear. This results in the opening of ion channels and initiation of a nerve signal to the brain.
Sodium ion channels in the tongue for sugar receptors open in response to binding of sucrose, allowing sodium concentration in the nerve cell to increase and initiate a nerve signal to the brain. In this case, the default for the gate is to be closed and it opens in response to binding of a ligand (sucrose).
In light sensing cells of the eye, calcium gates are open by default, but stimulation by light causes them to close, triggering a series of events that result in a signal being sent the brain about the perception of light. Thus, in this case, the stimulus (light) causes an open channel to close.
Moving the other direction, nerve signals originating in the brain travel to muscle tissue and through a complicated set of exchanges, result in the opening of calcium gates of muscle cells, increasing the concentration of calcium and stimulating muscular contraction (see HERE).
Voltage gated channels are essential for transmission of nerve signals, a process discussed in more depth HERE.
Ion movement through channels
The ability of ion channels to select against ions too large is intuitive - the size of the opening in the ion channel simply isn’t big enough for a larger ion to fit through the opening. Potassium, for example, passes through sodium channels rarely because the opening is too small.
Potassium channels that are selective for potassium ions must be big enough to allow potassium to enter, but if size were the only selection means, then sodium ions would also readily pass through potassium channels, since sodium ions (0.95 Å) are smaller than potassium ions (1.33 Å). In order for potassium channels to select against sodium ions and favor potassium ions, other considerations come into play.
Hydration shell
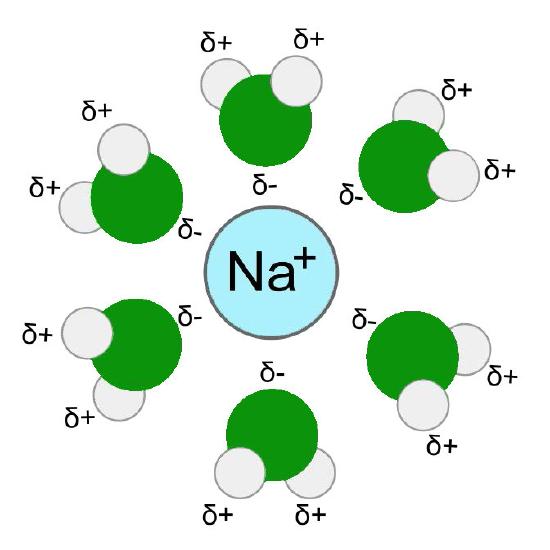
To understand this unique selectivity, it is important to understand how ions move through channels. Before an ion can pass through a channel, it must first be dissociated from (stripped of) the water molecules in its hydration shell - water molecules surrounding ions in aqueous solutions (Figure 3.32). This process requires an input of energy. The initial energy required to strip the water molecules from the hydration shell has been compared to the activation energy of an enzymatic reaction.
Comparable to enzymes
Just as enzymes lower the activation energy of enzymatic reactions and thus allow them to more readily occur, so too do channel proteins lower the energy requirements for a molecule to traverse a lipid bilayer. In the absence of the channel protein, the dehydration energy is mostly prohibitive for most polar molecules to occur, so very few make it across the lipid bilayer without the channel protein. This is why ion channel/transport proteins are so important to the cell.
After the water has been stripped, the ion can pass through the channel and when it arrives at the other side of the channel, the diffusing ion becomes rehydrated, thus regaining the energy that was required initially to strip away the water molecules from the ion.
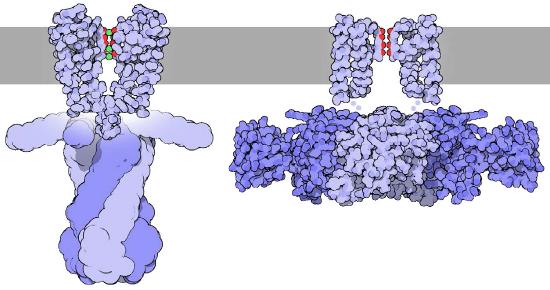
Selectivity of the potassium channel
The potassium channel (Figure 3.33) uses the dimensions of the potassium ion precisely to shepherd it through the channel. The sodium ion, which has different dimensions has a more difficult time making it through the channel despite its smaller size. The reason this is rooted in the energy required for dehydration.
For potassium ions, after the water has been stripped off, precisely positioned carbonyl groups along the channel help to stabilize the ion as it moves. The sodium ion, on the other hand is too small and does not make efficient connections with carbonyl groups and thus has a more difficult path. Because of this, the energy difference between dehydration and rehydration of a sodium ion in a potassium channel is energetically unfavorable (requires net input of energy) but the same process for a potassium ion is energetically favorable (results in a net gain of energy).
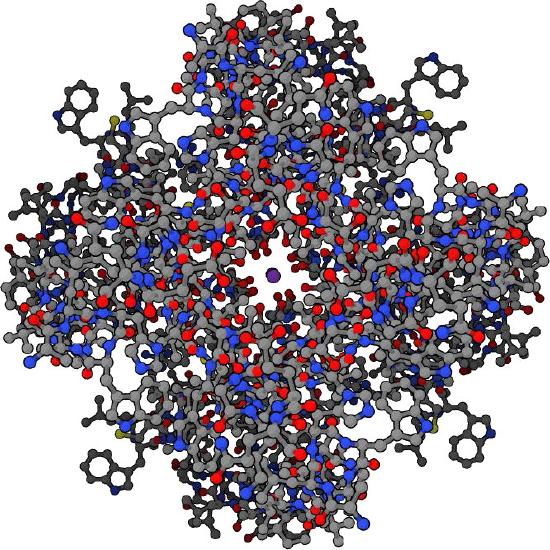
Movie 3.1 - Gramicidin A Wikipedia (animated gif, download to view)
Energy factor
Thus the selection in favor of potassium and against sodium ions in a potassium channel is based on energy, not physical size, whereas in the selection of sodium ions over potassium ions in a sodium channel, size is the primary consideration.
Ion balance
The movement of ions across a lipid bilayer is tightly regulated, and with good reason. Maintaining a proper balance of ions inside and outside of cells is important for maintaining osmotic balance. It is also important inside and outside of organelles like the mitochondria and chloroplasts for energy generation. If the ionic balance of a cell is sufficiently disturbed by an uncontrolled ionophore, a cell may die.
Gramicidin
Gramicidins (Movie 3.1) are antibiotic polypeptides synthesized by the soil bacterium known as Bacillus brevis. These small pentadecapeptides (15 amino acids) are synthesized by the bacterium to kill other bacteria.
When released by the Bacillus brevis, the gramicidins insert themselves in the membranes of Gram positive bacteria and allow the movement of sodium ions into the target cells, ultimately killing them. Gramicidins can also cause hemolysis in humans so they cannot be used internally, but instead are used topically.
Aquaporins
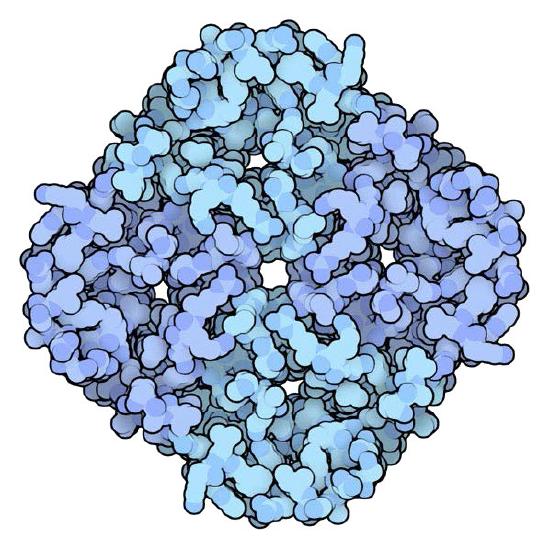
Aquaporins are pore-containing integral membrane proteins that selectively permit passage of water molecules in and out of the cell, while preventing ions and other solutes from moving (Figures 3.34 & 3.35). Some aquaporins called aquaglyceroporins, also transport other small uncharged entities, such as glycerol, ammonia, urea, and CO2, across the membrane,. The water pores are completely impermeable to charged molecules, such as protons, which is important for the preserving the membrane's electrochemical potential difference.

Porins
Porins are proteins containing a β-barrel structure that crosses the cell membrane/wall and acts as a pore/channel through which specific molecules diffuse. Porins are found in the outer membrane of Gram-negative bacteria and some Gram-positive bacteria, mitochondria, and chloroplasts.
Porins typically transport only one group of molecules or, in some cases, one specific molecule. Antibiotics, such as β-lactam and fluoroquinolone pass through porins to reach the cytosol of Gram-negative bacteria. Bacteria may develop resistance to these antibiotics when a mutation occurs to the porin involved that results in exclusion of the antibiotics that would otherwise pass through.
Transporter proteins
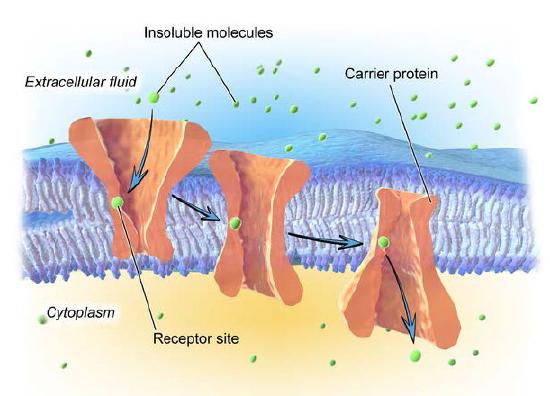
Not all facilitated transport occurs through ion channel proteins. Transporter proteins, as noted earlier (HERE and Figure 3.27) facilitate movement of materials across a lipid bilayer, but are slower than ion channels. Figure 3.36 illustrates a transporter protein in action. As can be seen, transporter proteins rely on a specific receptor site for proper recognition of the molecule to be moved.
Binding of the proper molecule causes a conformational change in the shape of the protein (an eversion) which results in a flipping of the open side of the protein to the other side of the lipid bilayer. In this way, the molecule is moved. Like ion channels, transporter proteins facilitate movement of materials in either direction, driven only by the concentration difference between one side and the other.
Active transport
All of the transport mechanisms described so far are driven solely by a concentration gradient - moving from higher concentrations in the direction of lower concentrations. These movements can occur in either direction and, as noted, result in equal concentrations on either side of the bilayer, if allowed to go to completion. Many times, however, cells must move materials against a concentration gradient and when this occurs, another source of energy is required. This process is known as active transport.
A good definition of active transport is that during active transport, at least one molecule is being moved against a concentration gradient. A common, but not exclusive, energy source is ATP (see Na+/K+ ATPase), but other energy sources are also employed. For example, the sodium-glucose transporter uses a sodium gradient as a force for actively transporting glucose into a cell. Thus, it is important to know that not all active transport uses ATP energy.
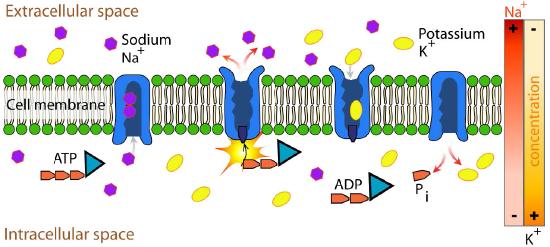
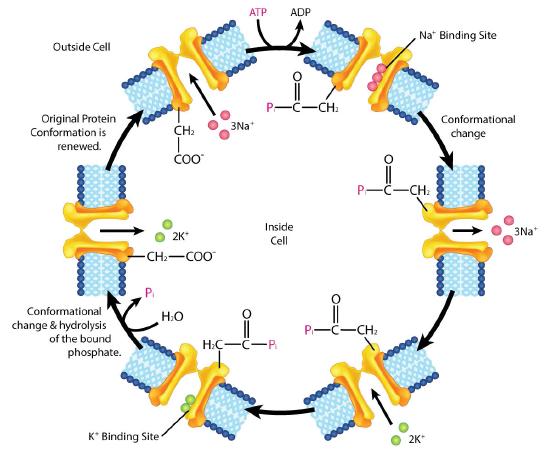
Na+/K+ ATPase
An important integral membrane transport protein is the Na+/K+ ATPase antiport (Figures 3.37 and 3.38), which moves three sodium ions out of the cell and two potassium ions into the cell with each cycle of action. In each case, the movement of ions is against the concentration gradient. Since three positive charges are moved out for each two positive charges moved in, the system is electrogenic.
The protein uses the energy released during ATP hydrolysis to create ion gradients that are important both in maintaining cellular osmotic pressure and (in nerve cells) for creating the sodium and potassium gradients necessary for signal transmission. Failure of the system to function results in swelling of the cell due to movement of water into the cell through osmotic pressure. The transporter expends about one fifth of the ATP energy of animal cells.
The cycle of action occurs as follows:
- Pump binds ATP followed by binding of 3 Na+ ions from cytoplasm of cell
- ATP hydrolysis results in phosphorylation of aspartate residue of pump. ADP is released
- Phosphorylated pump undergoes conformational change to expose Na+ ions to exterior of cell. Na+ ions are released.
- Pump binds 2 extracellular K+ ions.
- Pump dephosphorylates causing it to expose K+ ions to cytoplasm as pump returns to original shape.
- Pump binds 3 Na+ ions, binds ATP and releases 2 K+ ions to restart process
The Na+/K+ ATPase is classified as a P-type ATPase. This category of pump is notable for having a phosphorylated aspartate intermediate and is present across the biological kingdoms - bacteria, archaeans, and eukaryotes.
Na+/glucose transporter
Absorbing nutrients from the digestive system is necessary for animal life. The sodium/glucose transport protein is an electrogenic symporter that moves glucose into intestinal cells. It is found in the intestinal mucosa and the proximal tubule of the nephron of the kidney. The sodium/glucose transport system functions in the latter to promote reabsorption of glucose.
The pump works in conjunction with the Na+/K+ transport system. The gradient of sodium ions built up by the Na+/K+ pump is used as an energy source to drive movement of glucose into cells (see Figure 3.38). Use of an ion gradient established by a separate pump is known as secondary active transport. For intestinal mucosa, the pump transports glucose out of the gut and into gut cells. Later, the glucose is exported out the other side of the gut cells to the interstitial space for use in the body.
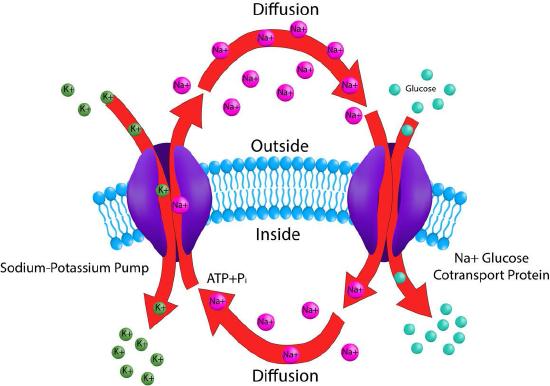
Not all transport proteins perform active transport.
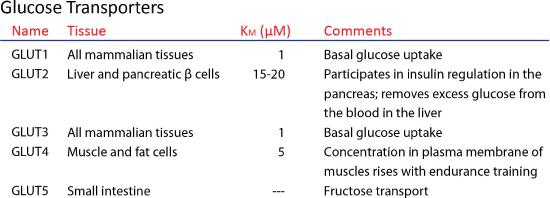
GLUTs
GLUTs (GLUcose Transport proteins) are uniport, type III integral membrane proteins that participate in the transport of glucose across membranes into cells. GLUTs are found in all phyla and are abundant in humans, with 12 GLUT genes. GLUT1, in erythrocytes is well-studied. Through GLUT 1, glucose enters and passes through it via facilitated diffusion at a rate that is 50,000 higher than in its absence. GLUTs of various types are found in different cells of the body. The one in red blood cells is known as GLUT 1 and has 12 membrane-spanning hydrophobic helices.
Though the structure of GLUT 1 is not known, it is speculated that the 12 helices form a chamber able to form hydrophilic bonds with glucose to facilitate its passage.
GLUT 1 levels in erythrocytes go up as glucose levels decrease and decrease when glucose levels go down. GLUT 1 can also transport ascorbate (vitamin C) in addition to glucose in mammals (such as humans) that do not produce their own vitamin C.
Glut 4
GLUT 4 is regulated by insulin and is found primarily in adipose and striated muscle tissue. Insulin alters intracellular trafficking pathways in response to increases in blood sugar to favor movement of various GLUT proteins (including GLUT 4) from intracellular vesicles to the cell membrane, thus stimulating uptake of the glucose. GLUT 4 is also found in the hippocampus where, if trafficking is disrupted, the result can be depressive behavior and cognitive dysfunction.
For all of the GLUT proteins, a key to keeping the glucose in the cell is phosphorylation of it by the glycolysis enzyme, hexokinase, in the cytoplasm. Phosphorylated molecules cannot enter GLUTs and don’t have an easy means of exiting the cell.
Note: while we consider each type of transport protein separately, they work together in the cell. Consider how the three transporters described above work together to get glucose you eat from your intestines into to your bloodstream.


