Comprehensive Homework 1 (Due 1/14/2019)
- Page ID
- 150533
Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Q1.1
Does a person with a mass of 50 kg climbing a height of 15 m do work? Explain your answer. Does that same person do work while descending a mountain?
Q1.2
Are the units for potential energy the same as the units for kinetic energy? Can an absolute value for potential energy be obtained (i.e., what is the zero for potential energy and does it matter for problems)? Explain your answer.
Q1.3
Decide whether the following are endothermic or exothermic processes
- water evaporates off a shower door
- an acid tablet being added to a pool and the surrounding water heats up
- \(NH_4Cl\) is dissolved in water and the solution cools
- the burning of a log in a campfire
Q1.4
Explain the difference between heat capacity and specific heat of a substance.
Q1.5
Calculate the heat capacity, in joules and in calories per degree, of the following:
- 28.4 g of water
- 1.00 oz of lead
Q1.6
How much heat, in joules and in calories, is required to heat a 28.4-g (1-oz) ice cube from −23.0 °C to −1.0 °C?
Q1.7
During the late spring, icebergs in the North Atlantic pose a hazard to shipping. To avoid them, ships travel routes that are about 30% longer. Many attempts have been made to destroy icebergs, including using explosives, torpedoes, and bombs. How much heat must be generated to melt 15% of a \(1.9 × 10^8\) kg iceberg? How many kilograms of TNT (trinitrotoluene, \(\ce{C7H5N3O6}\)) would be needed to provide enough energy to melt the ice? (The heat released for explosive decomposition of TNT is −1035.8 kJ/mol.)
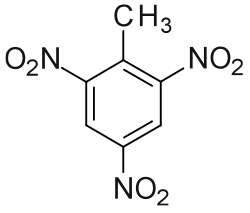
A topical question (ungraded): What property (or properties) makes a compound a good explosive?
Q1.8
200 g of ice cream at \(-16 ^oC\) is put on a 600 g glass plate at 25 ºC. At what temperature do they settle, assuming no heat flow to or from their surroundings. (Use 1.67 J/(g*C) for the ice cream and that glass has a specific heat of 0.742 J/(g*C)) The ice cream does not melt.

