7.6: ATP as Energy carrier
- Page ID
- 279631
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Cells couple the exergonic reaction of ATP hydrolysis with endergonic reactions to harness the energy within the bonds of ATP.
Learning Objectives
- Explain the role of ATP as the currency of cellular energy
ATP: Adenosine Triphosphate
Adenosine triphosphate (ATP) is the energy currency for cellular processes. ATP provides the energy for both energy-consuming endergonic reactions and energy-releasing exergonic reactions, which require a small input of activation energy. When the chemical bonds within ATP are broken, energy is released and can be harnessed for cellular work. The more bonds in a molecule, the more potential energy it contains. Because the bond in ATP is so easily broken and reformed, ATP is like a rechargeable battery that powers cellular process ranging from DNA replication to protein synthesis.
Molecular Structure
Adenosine triphosphate (ATP) is comprised of the molecule adenosine bound to three phosphate groups. Adenosine is a nucleoside consisting of the nitrogenous base adenine and the five-carbon sugar ribose. The three phosphate groups, in order of closest to furthest from the ribose sugar, are labeled alpha, beta, and gamma. Together, these chemical groups constitute an energy powerhouse. The two bonds between the phosphates are equal high-energy bonds (phosphoanhydride bonds) that, when broken, release sufficient energy to power a variety of cellular reactions and processes. The bond between the beta and gamma phosphate is considered “high-energy” because when the bond breaks, the products [adenosine diphosphate (ADP) and one inorganic phosphate group (Pi)] have a lower free energy than the reactants (ATP and a water molecule). ATP breakdown into ADP and Pi is called hydrolysis because it consumes a water molecule (hydro-, meaning “water”, and lysis, meaning “separation”).

ATP Hydrolysis and Synthesis
ATP is hydrolyzed into ADP in the following reaction:
ATP+H2O→ADP+Pi+free energy
Like most chemical reactions, the hydrolysis of ATP to ADP is reversible. The reverse reaction combines ADP + Pi to regenerate ATP from ADP. Since ATP hydrolysis releases energy, ATP synthesis must require an input of free energy.
ADP is combined with a phosphate to form ATP in the following reaction:
ADP+Pi+free energy→ATP+H2O
The phosphorylation (or condensation of phosphate groups onto AMP) is an endergonic process. By contrast, the hydrolysis of one or two phosphate groups from ATP, a process called dephosphorylation, is exergonic. Why? Let's recall that the terms endergonic and exergonic refer to the sign on the difference in free energy of a reaction between the products and reactants, ΔG. In this case we are explicitly assigning direction to the reaction, either in the direction of phosphorylation or dephosphorylation of the nucleotide. In the phosphorylation reaction the reactants are the nucleotide and an inorganic phosphate while the products are a phosphorylated nucleotide and WATER. In the dephosphorylation/hydrolysis reaction, the reactants are the phosphorylated nucleotide and WATER while the products are inorganic phosphate and the nucleotide minus one phosphate.
"High-Energy" bonds
What about the term "high-energy bonds" that we so often hear associated with ATP? If there is nothing "special" about the bonds in ATP, why do we always hear the term "high-energy bonds" associated with the molecule? The answer is deceptively simple. In biology the term "high-energy bond" is used to describe an exergonic reaction involving the hydrolysis of the bond in question that results in a "large," negative change in free energy. Remember that this change in free energy does not only have to do with the bond in question but rather the sum of all bond rearrangements in the reaction. What constitutes a large change? It is a rather arbitrary assignment usually associated with an amount of energy associated with the types of anabolic reactions we typically observe in biology. If there is something special about the bonds in ATP, it is not uniquely tied to the free energy of hydrolysis, as there are plenty of other bonds whose hydrolysis results in greater negative differences in free energy.
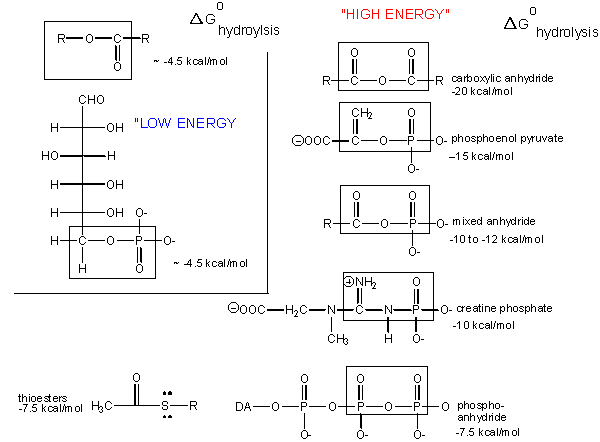
Figure 2. The free energy of hydrolysis of different types of bonds can be compared to that of the hydrolysis of ATP. Source: http://bio.libretexts.org/Core/Biochemistry/Oxidation_and_Phosphorylation/ATP_and_Oxidative_Phosphorylation/Properties_of_ATP
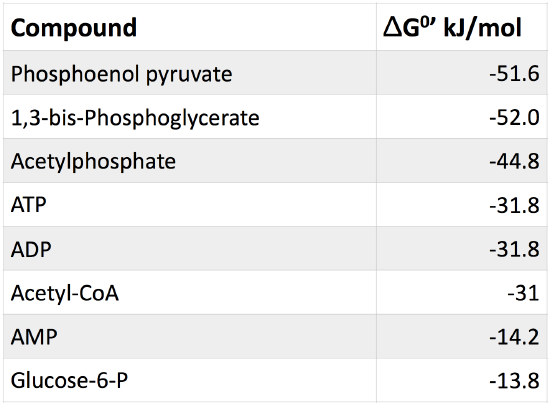
Table 1. Table of common cellular phosphorylated molecules and their respective free energies of hydrolysis, under physiological conditions.
ATP and Energy Coupling
Exactly how much free energy (∆G) is released with the hydrolysis of ATP, and how is that free energy used to do cellular work? The calculated ∆G for the hydrolysis of one mole of ATP into ADP and Pi is −7.3 kcal/mole (−30.5 kJ/mol). However, this is only true under standard conditions, and the ∆G for the hydrolysis of one mole of ATP in a living cell is almost double the value at standard conditions: 14 kcal/mol (−57 kJ/mol).
ATP is a highly unstable molecule. Unless quickly used to perform work, ATP spontaneously dissociates into ADP + Pi, and the free energy released during this process is lost as heat. To harness the energy within the bonds of ATP, cells use a strategy called energy coupling.
Cells couple the exergonic reaction of ATP hydrolysis with the endergonic reactions of cellular processes. For example, during cellular metabolic reactions, or the synthesis and breakdown of nutrients, certain molecules must be altered slightly in their conformation to become substrates for the next step in the reaction series. In the very first steps of cellular respiration, glucose is broken down through the process of glycolysis. ATP is required for the phosphorylation of glucose, creating a high-energy but unstable intermediate. This phosphorylation reaction causes a conformational change that allows enzymes to convert the phosphorylated glucose molecule to the phosphorylated sugar fructose. Fructose is a necessary intermediate for glycolysis to move forward. In this example, the exergonic reaction of ATP hydrolysis is coupled with the endergonic reaction of converting glucose for use in the metabolic pathway.
The cycling of ATP pools
Estimates for the number of ATP molecules in a typical human cell range from ~3x107 (~5x10-17 moles ATP/cell) in a white blood cell to 5x109 (~9x10-15 moles ATP/cell) in an active cancer cell. While these numbers might seem large, and already amazing, consider that it is estimated that this pool of ATP turns over (becomes ADP and then back to ATP) 1.5 x per minute. Extending this analysis yields the estimate that this daily turnover in your body, amounts to roughly the equivalent of one body weight of ATP getting turned over per day. That is, if no turnover/recycling of ATP happened, it would take 1 body weights worth of ATP for the human body to function - hence our previous characterization of ATP as a "short term" energy transfer device for the cell.
While the pool of ATP/ADP may be recycled, some of the energy that is transferred in the many conversions between ATP, ADP and other biomolecules is also transferred to the environment. In order to maintain cellular energy pools (that is, keep the concentration of ATP (vs. ADP) up to the level the cell needs) energy must transfer in from the environment as well. Where does this energy come from? The answer depends a lot on where energy is available and what mechanisms have evolved to transfer energy from the environment to molecular carriers like ATP. In nearly all cases, however, the mechanism of transfer includes some form of redox chemistry. In this and the sections that follow we will be studying some critical examples of energy transfer from the environment, key types of chemistry and biological reactions involved in this process, and some of the key biological reactions and cellular components associated with energy flow between different parts of the living system. We focus first on reactions involved in the (re)generation of ATP in the cell (not those involved in the creation of the nucleotide per se but rather those associated with the transfer of phosphates onto AMP and ADP- in other words, "recharging" of ADP/ATP).
How do cells generate ATP?
A variety of mechanisms have emerged over the 3.25 billion years of evolution to create ATP from ADP and AMP. The majority of these mechanism are modifications on two basic classes of mechanisms known as Substrate Level Phosphorylation (SLP) and oxidative phosphorylation. These topics are substantive enough that they will be discussed in detail in the next few modules. Both mechanisms rely on biochemical reactions that transfer energy from some energy source to ADP or AMP, to synthesize ATP.
Key Points
- Adenosine triphosphate is composed of the nitrogenous base adenine, the five-carbon sugar ribose, and three phosphate groups.
- ATP is hydrolyzed to ADP in the reaction ATP+H2O→ADP+Pi+ free energy; the calculated ∆G for the hydrolysis of 1 mole of ATP is -57 kJ/mol.
- ADP is combined with a phosphate to form ATP in the reaction ADP+Pi+free energy→ATP+H2O.
- The energy released from the hydrolysis of ATP into ADP is used to perform cellular work, usually by coupling the exergonic reaction of ATP hydrolysis with endergonic reactions.
- Sodium-potassium pumps use the energy derived from exergonic ATP hydrolysis to pump sodium and potassium ions across the cell membrane while phosphorylation drives the endergonic reaction.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- OpenStax College, Biology. October 21, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44427/latest...ol11448/latest. License: CC BY: Attribution
- OpenStax College, Biology. October 26, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44427/latest...ol11448/latest. License: CC BY: Attribution
- hydrolysis. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/hydrolysis. License: CC BY-SA: Attribution-ShareAlike
- exergonic. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/exergonic. License: CC BY-SA: Attribution-ShareAlike
- endergonic. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/endergonic. License: CC BY-SA: Attribution-ShareAlike
- Boundless. Provided by: Boundless Learning. Located at: www.boundless.com//biology/de...nergy-coupling. License: CC BY-SA: Attribution-ShareAlike
- free energy. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/free_energy. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Biology. October 26, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44427/latest...ol11448/latest. License: CC BY: Attribution
- OpenStax College, Biology. October 26, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44427/latest...ol11448/latest. License: CC BY: Attribution
- ATP. BIS 2A: Introductory Biology (Britt) Course. University of California Davis (Sept. 23, 2020) . Located at: https://bio.libretexts.org/Courses/University_of_California_Davis/BIS_2A%3A_Introductory_Biology_(Singer)_II/MASTER_RESOURCES/ATP*%23. License: CC BY-NC-SA 3.0.


