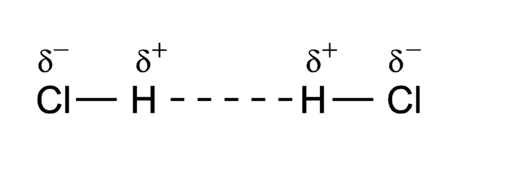1.2: Basic Chemistry
- Page ID
- 233971
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Source: BiochemFFA_1_2.pdf. The entire textbook is available for free from the authors at http://biochem.science.oregonstate.edu/content/biochemistry-free-and-easy
“Organic chemistry is the chemistry of carbon compounds. Biochemistry is the chemistry of carbon compounds that crawl” -Michael Adams.
To understand biochemistry, one must possess at least a basic understanding of organic and general chemistry. In this brief section, we will provide a rapid review of the simple concepts necessary to understand cellular chemistry. Chemistry is chemistry, whether in a cell or outside it, but biological chemistry is a particular subset of organic chemistry that often involves enormous macromolecules, and that happens in the aqueous environment of the cell.
Chemical bonds: ionic vs covalent bonds
Atoms consist of a nucleus (formed by neutrons and protons) plus electrons moving around the nucleus. In a neutral atom (element), the number of electrons and protons are equal (an atom has zero net charge). The octet rule stipulates that atoms react to form compounds trying to emulate the number of electrons of the noble gas (group 18) closer to their location on the period table; that is, atoms like to be surrounded by 8 electrons (helium being the exception with only two electrons). In order to fulfill the octet rule, atoms have two options: they can either transfer electrons or share electrons.
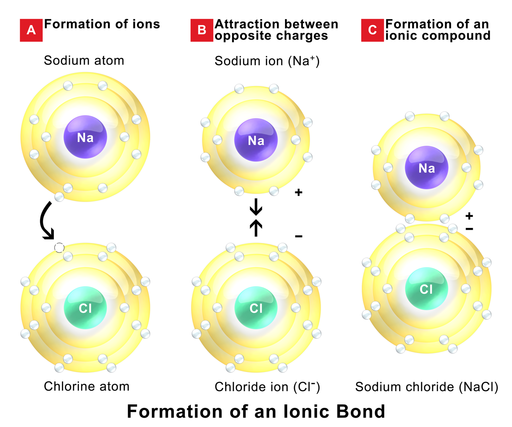
An ionic bond happens when an metallic element (to the left on the period table) transfers an electron to a non-metallic element (to the right on the period table) forming a cation and an anion, respectively. This ions have opposite charges, so they attract mutually resulting in the formation of an ionic compound. Because of the electrostatic attraction between the ionic net charges, ionic compounds have very high melting and boiling points. and they form crystals at room temperature. The formation of sodium chloride is a typical example of an ionic compound.
Figure 1.2.1. Formation of an ionic compound. Image by BruceBlaus, CC BY-SA 4.0, via Wikimedia Commons
Another important characteristic of ionic compounds is that many are soluble in water, due to an intermolecular interaction called ion-dipole (see below). When the ions in an ionic compound get in contact with a polar solvent like water, the ions can interact with this polar solvent, and as the consequence the ionic compound desintegrates forming ions in solutions. This process is called dissociation. Because the ions in solution are free to move, solutions of ionic compounds can conduct the electricity, so they are called electrolytes. Highly polarized covalent compounds (such as HCl) can also undergo dissociation in water. In this case, the process is called ionization. In ionization, a hydrogen ion (H+) leaves behind its electron as it exits (leaving behind a negative charge on the Cl-).
A covalent compound happens when a nonmetallic element reacts with another nonmetallic element, resulting in an electron sharing to fulfill the octet rule. For example, two atoms of Fluorine can share one pair of electrons resulting in the formation of a F2 molecule. Covalent compounds tend to have lower melting points and lower points than ionic compounds and their physical properties (melting point, boiling point, solubility, etc) depend on the strength of the forces with which different molecules interact with each other. These forces are called intermolecular interactions, which in turn, are related to the electronegativity difference between atoms in a covalent bond (and molecular geometry)
Figure 1.2.2. Formation of covalent bond in fluorine molecule. Image by Jacek FH, CC BY-SA 3.0, via Wikimedia Commons.
Electronegativity
Electronegativity is a measure of the affinity a nucleus has for outer shell electrons (Table 1.2). High electronegativity corresponds to high affinity for electrons. Electrons in a covalent bond are held closer to the nucleus with a greater electronegativity compared to a nucleus with lower electronegativity.
| Table 1.2. Electronegativity of various atoms | |
| Oxygen | 3.5 |
| Nitrogen | 3.0 |
| Fluorine | 3.9 |
| Chlorine | 3.1 |
| Hydrogen | 2.1 |
| Carbon | 2.5 |
For example, in a molecule of HCl, with hydrogen covalently bonded to chlorine, the electrons are “pulled” toward the chlorine, which is more electronegative. Because of this, there is a slightly greater negative charge near the chlorine atom, compared to the hydrogen (which, correspondingly has a slightly higher positive charge). This unequal charge distribution sets up a permanent dipole, with one side being somewhat negative and the other somewhat positive. Because of this, the molecule is described as polar.
Intermolecular Forces
As mentioned above, covalent molecules can interact with other molecules through intermolecular forces. The nature of these intermolecular interactions determines many of the properties of covalent compounds. Intermolecular forces are always weaker than chemical bonds.
For example, the permanent dipoles in H-Cl can interact through dipole-dipole interactions. This interaction is present between all polar covalent molecules, and it is due to the electrostatic attraction between the partial charges in the permanent dipoles.
Figure 1.2.3. Dipole-Dipole interaction in hydrogen chloride. Image by P.wormer, CC BY-SA 3.0, via Wikimedia Commons
For the most electronegative elements on the period table, that is fluorine, oxygen, and nitrogen (FON), when they are directly attached to hydrogen, the intensity of the electrostatic attraction is so intense that reserves its unique denomination. This intermolecular interaction is known as hydrogen bond. Hydrogen bond is the main type of intermoelcular forces present in HF, NH3, and H2O. Hydrogen bonds between water molecules are the result of the attraction of the partial positive and partial negative charges on different water molecules (Figure 1.2.4). Hydrogen bonds can also form between hydrogens with a partial positive charge and other strongly electronegative atoms, like nitrogen, with a partial negative charge. It is important to remember that hydrogen bonds are interactions between molecules (or parts of molecules) and are not bonds between atoms, like covalent or ionic bonds.
Figure 1.2.4. Hydrogen bonds (dotted lines) between water molecules. Image by Qwerter at Czech wikipedia: Qwerter. Transferred from cs.wikipedia to Commons by sevela.p. Translated to english by by Michal Maňas (User:snek01). Vectorized by Magasjukur2, Public domain, via Wikimedia Commons
The presence of permanent dipoles in polar molecules like water explains the solubility of ionic compounds in this solvent. The net charges present in ionic compounds (cations with positive charge and anions with a negative charge) can interact very effectively with the partial charges present in the dipole molecules through an interaction known as ion-dipole. This interaction is strong enough that many ionic compounds are soluble in water, although sometimes the interaction between teh ions in teh crystal are stronger than the ion-dipoles forces, so not every ionic compound is soluble in water.
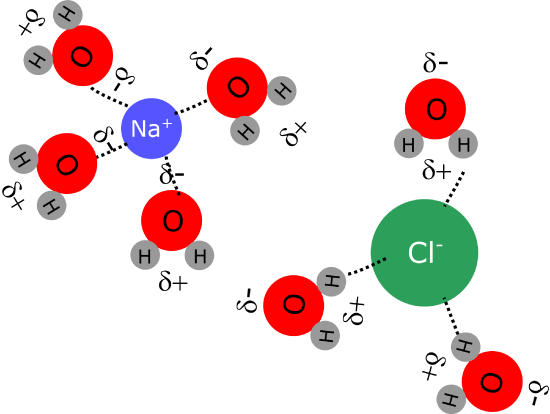
Figure 1.2.5. Ion dipole interactions between ions in sodium chloride and water molecules.
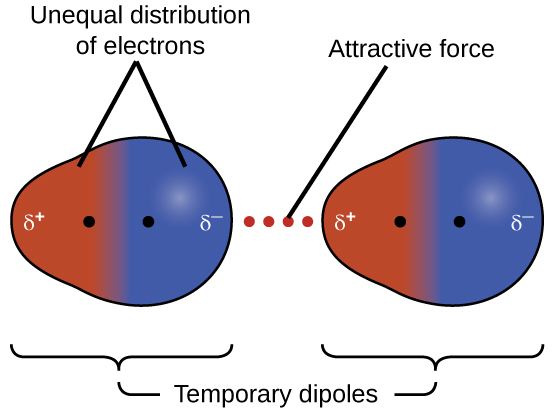
Bonds between hydrogen and carbon do not form significant partial charges because the electronegativities of the two atoms are similar. Consequently, molecules containing many carbon-hydrogen bonds will not form hydrogen bonds and therefore, do not mix well with polar solvents water. These compounds for which the difference of electronegativity between atoms is zero (or almost zero) are known as non-polar covalent compounds. Examples are F2, O2, CO2 (consider the linear molecular geometry here), or CH4. In non-polar compounds, the only possible intermolecular forces result from the mobility of electrons that might generate an uneven distribution of charge, with one atom becoming temporarily more negatively charged and another atom becoming more positively charged. This type of dipoles is very short-lived, and the attraction that results from these dipoles is very weak. This interaction is called London Dispersion Forces (LDF). Although London dispersion forces are weak, they increase with the number of atoms, so large molecules can exhibit quite strong LDF.
Figure 1.2.6. Temporary dipoles and London Dispersion forces. Image by OpenStax, CC BY 4.0, via Wikimedia Commons
Non-polar molecules with LDF are called hydrophobic because they do not mix well with water. Other polar compounds with the ability to dissolve in water are called hydrophilic. Molecules possessing both characteristics are called amphiphilic.
In compounds with similar molecular mass, the strength of these intermolecular forces decree in the following order:
ion-dipole > hydrogen bond > dipole-dipole > London Dispersion Forces
Functional groups
Organic chemistry os the study of carbon-containing compounds. These compounds are classified according to particular structural features called functional groups. Figure 1.2.7 shows the various organic functional groups common in biochemistry. You will encounter these functional groups as you study the biosynthetic and breakdown pathways that build and recycle the chemical compounds of which cells are made. In addition to knowing the names and structures of these groups, students need a basic understanding of covalent and ionic bonds.
Figure 1.2.7. Important functional groups in biochemistry Image by Aleia Kim
Covalent bonds, as you know, are the result of sharing of electrons between two atoms. Ionic bonds, by contrast, are formed when one atom donates an electron to another, such as in the formation of sodium chloride. Single covalent bonds can rotate freely, but double bonds cannot. Single bonds around a carbon atom are arranged in a tetrahedron with bond angles of 109.5° relative to each other, with the carbon at the center (Figure 1.19). Double bonded carbons create a planar structure with bond angles typically of about 120°
Oxidation/reduction in organic molecules.
Oxidation involves loss of electrons (oxidation number increases) and reduction results in the gain of electrons (oxidation number decreases). Oxidation reactions tend to release energy and are a source off energy. Every oxidation is always accompanied by a reduction, so these reactions are called redox reactions. This definition is very useful when dealing with inorganic compounds. However, most molecules encountered in biological systems are organic. When dealing with organic molecules, we need an easier to identify oxidation and reduction rather than counting oxidation numbers. For organic molecules, oxidation reactions can be understood as the gain of oxygen or the loss of hydrogen atoms. On the other hand, reduction reactions can be understood as the gain of hydrogen atoms, or the loss of oxygen.
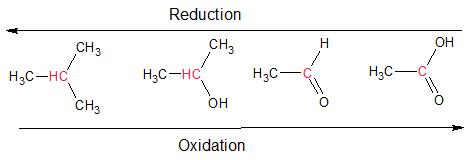
Figure 1.2.8. Redox reactions in organic chemistry. The carbon indicated in red is oxidized from left to right, and reduced from right to left.
Stereochemistry
A carbon has the ability to make four single bonds (forming a tetrahedral structure) and if it bonds to four different chemical groups, their atoms can be arranged around the carbon in two different ways, giving rise to enantiomers (Figure 1.2.9). Each carbon with such a property is referred to as an stereogenic center or chiral carbon. The property of handedness only occurs when a carbon has four different groups bonded to it. Enantiomers are stereoisomers that constitute mirror images from each other. Enantiomers have the same chemical and physical properties, but they differ in their biological properties. Enzymes have very specific 3-D structures, so they can differentiate between stereoisomers or enantiomers. By contrast, molecules made chemically (not using enzymes) end up with equal amounts of both enantiomers, called a racemic mix.
Figure 1.2.9. Enantiomers of lactic acid
Gibbs free energy
Chemical reactions are accompanied by energy changes. That energy can represent changes in heat (enthalpy, H) or disorder (entropy, S). In terms of enthalpy, a chemical reaction can be accompanied by a release of heat (ΔH<0) or absorption of heat (ΔH>0). The former is an exothermic reaction, and the latter is an endothermic reaction. In terms of entropy, a reaction can be accompanied by an increase (ΔS>0) or decrease of disorder (ΔS<0)
The total energy change in a chemical reaction can be expressed in terms of its free energy change, also known as Gibbs energy (G).
\[ΔG = ΔH - TΔS\]
The Gibbs free energy calculation allows us to determine whether a reaction will be spontaneous, by taking into consideration two factors, change in enthalpy (ΔH) and change in entropy (ΔS). The free energy change ΔG for a reaction equal to the enthalpy change (\(ΔH\)) minus the absolute temperature (T) times the entropy change (ΔS):
\[ΔG = ΔH - TΔS\]
A negative \(ΔG\) corresponds to release of free energy. Reactions that release energy are exergonic, whereas those that absorb energy are called endergonic. Exergonic reactions are spontaneous, while endergonic reactions are non-spontaneous.
The biological standard Gibbs free energy change (ΔG°’) corresponds to the ΔG for a process under standard conditions of temperature, pressure, and at pH = 7. For a reaction
\[aA + bB \rightleftharpoons cC + dD,\]
If a process has a \(ΔG = Z\) and a second process has a \(ΔG = Y\), then if the two processes are linked, \(ΔG\) and \(ΔG^{o \prime} \) values for the overall reaction will be the sum of the individual ΔG and ΔG°’ values.
\[ΔG_{total} = ΔG_1+ ΔG_2 = Z + Y\]
\[ΔG^{o \prime }_{total} = ΔG_1^{o \prime}+ ΔG_2^{o\prime}\]
Reversible and irreversible reactions
There two types of chemical reactions. Irreversible reactions are those for which the conversion of reactants into products is close to 100%, meaning that after teh reaction has taken place, almost no reactants remain and all we find in the reaction container are products. These reactions are indicated by a single reaction arrow:
\[aA + bB →cC + dD,\]
Irreversible reactions are difficult to undo. The same reactioncannot be used to convert product into reactants.
The other type of chemical reactions are reversible. For a reversible reaction, we use a double arrow to indicate that reactants react forming products, but at the same time, products can also react regenerating the reactants:
\[aA + bB \rightleftharpoons cC + dD,\]
After a certain time, a container in which a reversible reaction takes place reaches a situation in which the concentration of reactants and product remain constant, since the rate of the forward reactions the rate of the reverse reactions. When this happens, we say that the system has reached equilibrium, and we can define the equilibrium constant, \(K_{eq}\), which is equal to:
\[K_{eq} = \dfrac{ [C]^c_{eq} [D]^d_{eq}}{[A]^a_{eq} [B]^b_{eq}}\]
where \(a\), \(b\), \(c\), and \(d\) are integers in the balanced equation. Large values of \(K_{eq}\) correspond to favorable reactions (more C and D produced than A and B) and small values of \(K_{eq}\) mean the opposite. At equilibrium,
\[ΔG^{o\prime} = -RT \ln K_{eq}\]
Le Chatelier's principle establishes that a reaction at equilibrium can be shifted towards reactants or products by changing the reaction conditions, such as adding products or reactants, or removing products or reactants. In other words, reversible reactions can favor the formation of reactants or products, depending on the situation.
Both types of reactions, reversible and irreversible, can be found in biological systems, and therefore, they play a crucial role in maintaining and regulating the metabolic balance.
Reaction rates and catalysis
The rate of a chemical reaction depends on factors such as the temperature and the concentration of reactants. Increasing the temperature or the concentration of reactants can increase the reaction rate. The main factor regulating the rate of a chemical reaction is its activation energy. Reactions with high activation energy are slower than reactions with low activation energy, independently if the reaction is very spontaneous or not. Therefore, reducing the activation energy is another way by which we can increase reaction rate. A substance capable of reducing the activation energy for a reaction is called a catalyst. Because catalysts remain unchanged at the end of a reaction, a single catalyst molecule can be reused for many reaction cycles. Proteins that catalyze reactions in cells are called enzymes, while ribozymes are RNA molecules that act as catalysts.