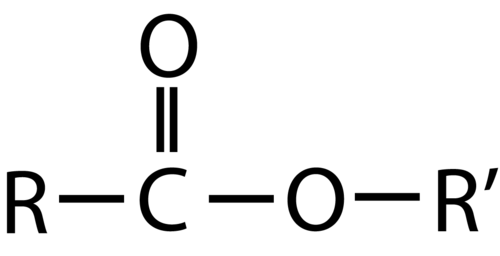2.4: Chemical Properties of Carboxylic Acids II- Formation of Esters
- Page ID
- 227640
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Describe the structure and properties of esters.
- Name common esters.
Formation of Esters: The Sweet Smell of RCOOR'
An ester is an organic compound that is a derivative of a carboxylic acid in which the hydrogen atom of the hydroxyl group has been replaced with an alkyl group. The structure is the product of a carboxylic acid (the \(\ce{R}\)-portion) and an alcohol (the \(\ce{R'}\)-portion). The general formula for an ester is shown below.
The \(\ce{R}\) group can either be a hydrogen or a carbon chain. The \(\ce{R'}\) group must be a carbon chain since a hydrogen atom would make the molecule a carboxylic acid.
Esters are produced by the reaction of acids with alcohols. For example, the ester ethyl acetate, CH3CO2CH2CH3, is formed when acetic acid reacts with ethanol:
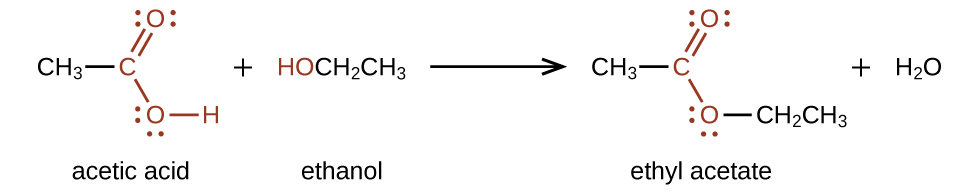
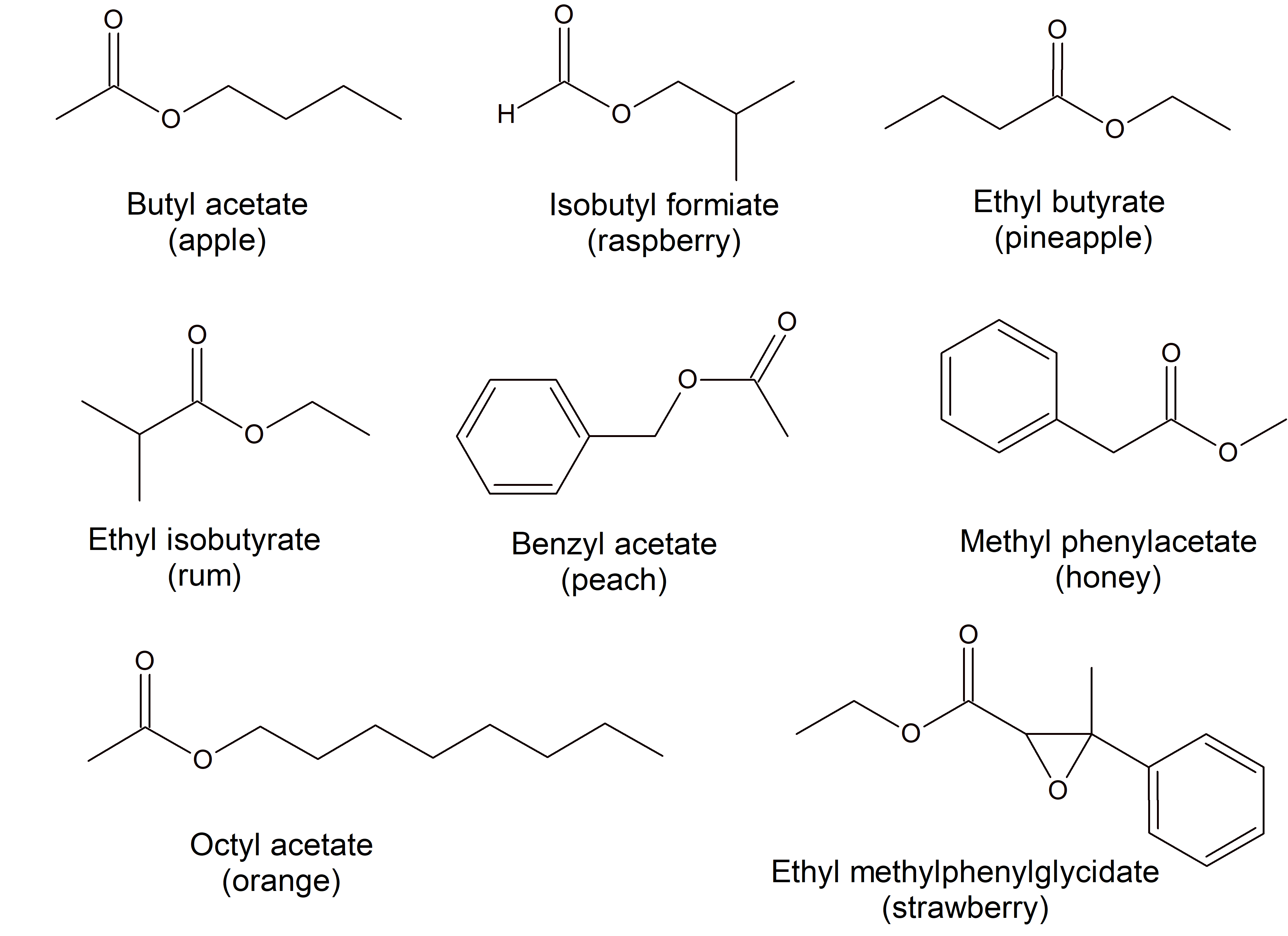 Figure \(\PageIndex{3}\) Esters are responsible for the odors associated with various plants and their fruits.
Figure \(\PageIndex{3}\) Esters are responsible for the odors associated with various plants and their fruits.Chemistry Is Everywhere: Esters, Fragrances, and Flavorings
Esters are very interesting compounds, in part because many have very pleasant odors and flavors. (Remember, never taste anything in the chemistry lab!) Many esters occur naturally and contribute to the odor of flowers and the taste of fruits. Other esters are synthesized industrially and are added to food products to improve their smell or taste; it is likely that if you eat a product whose ingredients include artificial flavorings, those flavorings are esters. Here are some esters and their uses, thanks to their odors, flavors, or both:
| Ester | Tastes/Smells Like | Ester | Tastes/Smells Like |
|---|---|---|---|
| allyl hexanoate | pineapple | isobutyl formate | raspberry |
| benzyl acetate | pear | isobutyl acetate | pear |
| butyl butanoate | pineapple | methyl phenylacetate | honey |
| ethyl butanoate | banana | nonyl caprylate | orange |
| ethyl hexanoate | pineapple | pentyl acetate | apple |
| ethyl heptanoate | apricot | propyl ethanoate | pear |
| ethyl pentanoate | apple | propyl isobutyrate | rum |
Finally, the ether functional group is an
Among the most important of the natural esters are fats (such as lard, tallow, and butter) and oils (such as linseed, cottonseed, and olive oils), which are esters of the trihydroxyl alcohol glycerine, C3H5(OH)3, with large carboxylic acids, such as palmitic acid, CH3(CH2)14CO2H, stearic acid, CH3(CH2)16CO2H, and oleic acid, \(\mathrm{CH_3(CH_2)_7CH=CH(CH_2)_7CO_2H}\). Oleic acid is an unsaturated acid; it contains a \(\mathrm{C=C}\) double bond. Palmitic and stearic acids are saturated acids that contain no double or triple bonds.
Note
Fats and vegetable oils are esters of long-chain fatty acids and glycerol. Esters of phosphoric acid are of the utmost importance to life.
Esters are common solvents. Ethyl acetate is used to extract organic solutes from aqueous solutions—for example, to remove caffeine from coffee. It also is used to remove nail polish and paint. Cellulose nitrate is dissolved in ethyl acetate and butyl acetate to form lacquers. The solvent evaporates as the lacquer “dries,” leaving a thin film on the surface. High boiling esters are used as softeners (plasticizers) for brittle plastics.
Summary
- An ester has an OR group attached to the carbon atom of a carbonyl group.
- Fats and vegetable oils are esters of long-chain fatty acids and glycerol.
- Esters occur widely in nature and generally have pleasant odors and are often responsible for the characteristic fragrances of fruits and flowers.
Contributors and Attributions
- Libretext : The Basics of GOB Chemistry (Ball et al.)
- TextMap: Beginning Chemistry (Ball et al.)
- OpenSTAX
- Carboxylic Acids and Esters. (2020, August 13). Retrieved May 22, 2021, from https://chem.libretexts.org/@go/page/153839