2.19: Reaction Mechanism for Free-Radical Halogenation of Alkanes
- Page ID
- 221769
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Alkanes (the most basic of all organic compounds) undergo very few reactions. One of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen to form a haloalkane. This reaction is very important in organic chemistry because it opens a gateway to further chemical reactions.
Introduction
While the reactions possible with alkanes are few, there are many reactions that involve haloalkanes. In order to better understand the mechanism (a detailed look at the step by step process through which a reaction occurs), we will closely examine the chlorination of methane. When methane (CH4) and chlorine (Cl2) are mixed together in the absence of light at room temperature nothing happens. However, if the conditions are changed, so that either the reaction is taking place at high temperatures (denoted by Δ) or there is ultra violet irradiation, a product is formed, chloromethane (CH3Cl).
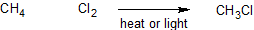
Energetics
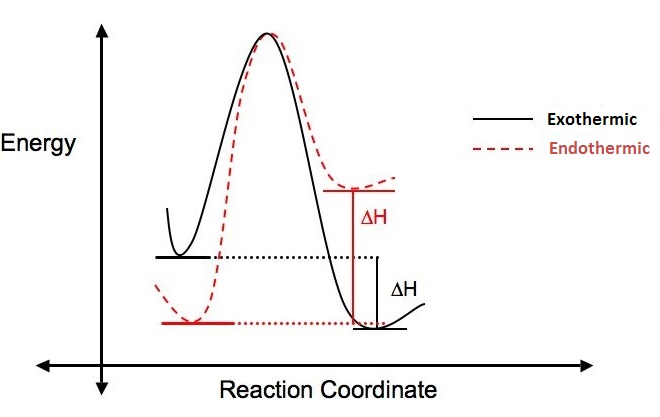
Why does this reaction occur? Is the reaction favorable? A way to answer these questions is to look at the change in enthalpy (\(\Delta{H}\)) that occurs when the reaction takes place.
ΔH = (Energy put into reaction) – (Energy given off from reaction)
If more energy is put into a reaction than is given off, the ΔH is positive, the reaction is endothermic and not energetically favorable. If more energy is given off in the reaction than was put in, the ΔH is negative, the reaction is said to be exothermic and is considered favorable. The figure below illustrates the difference between endothermic and exothermic reactions.
ΔH can also be calculated using bond dissociation energies (ΔH°):
\[\Delta{H} = \sum \Delta{H^°} \text{ of bonds broken} - \sum \Delta{H^°} \text{ of bonds formed}\]
Let’s look at our specific example of the chlorination of methane to determine if it is endothermic or exothermic:
.jpg?revision=2)
Since, the ΔH for the chlorination of methane is negative, the reaction is exothermic. Energetically this reaction is favorable. In order to better understand this reaction, we need to look at the mechanism ( a detailed step by step look at the reaction showing how it occurs) by which the reaction occurs.
Radical Chain Mechanism
The reaction proceeds through the free radical chain mechanism. A free radical is a compound that contains an unpaired valence electron (indicated as a single dot in a Lewis structure). Because of their incomplete octet, free radicals are very unstable and chemically reactive (there are a few minor exceptions). Reaction mechanisms involving free radicals are characterized by three steps: initiation, propagation, and termination. Initiation requires an input of energy (usually light or UV radiation) to generate the initial free radical, but after this initial free radical is generated, the reaction is self-sustaining. The first propagation step uses up one of the products from initiation, and the second propagation step makes another one, thus the cycle can continue indefinitely. This is why the mechanism is said to be a "chain reaction:" once the initiation step has started, the reaction will continue until the termination step is completed.
Step 1: Initiation
Initiation breaks the bond between the chlorine molecule (Cl2). For this step to occur energy must be put in, this step is not energetically favorable. Thererore, the reaction needs some form of energy such as heat or UV radiation to act as a catalyst. After this step, the reaction can occur continuously (as long as reactants provide) without input of more energy. It is important to note that this part of the mechanism cannot occur without some external energy input, through light or heat.
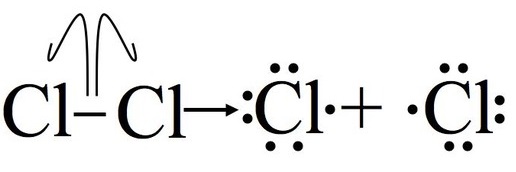
Step 2: Propagation
The next two steps in the mechanism are called propagation steps. In the first propagation step, a chlorine radical combines with a hydrogen on the methane. This gives hydrochloric acid (HCl, the inorganic product of this reaction) and the methyl radical. In the second propagation step more of the chlorine starting material (Cl2) is used, one of the chlorine atoms becomes a radical and the other combines with the methyl radical.
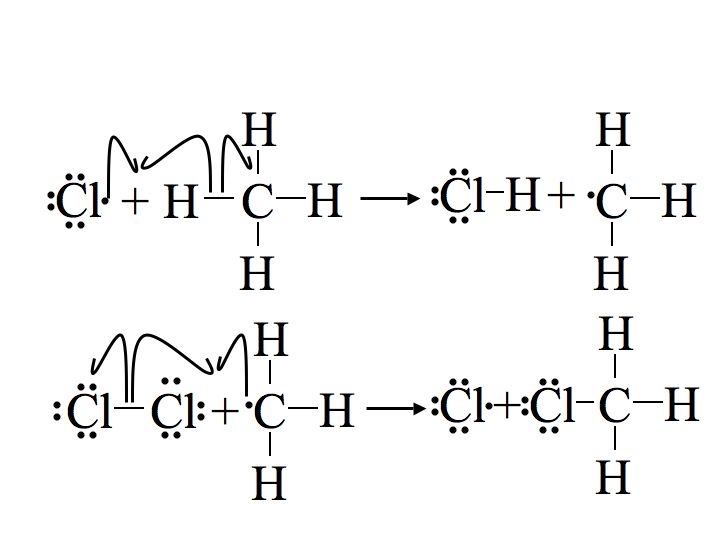
The first propagation step is endothermic, meaning it takes in heat (requires 2 kcal/mol) and is not energetically favorable. In contrast the second propagation step is exothermic, releasing 27 kcal/mol. Once the reaction is initiated, the exothermic energy released from the second propagation step provides the activation energy for the first propagation step creating a cyclic chain reaction following Le Chatelier's principle until termination.
.gif?revision=2)
Step 3: Termination
In the termination steps, all the remaining radicals combine (in all possible manners) to form more product (CH3Cl), more reactant (Cl2) and even combinations of the two methyl radicals to form a side product of ethane (CH3CH3).
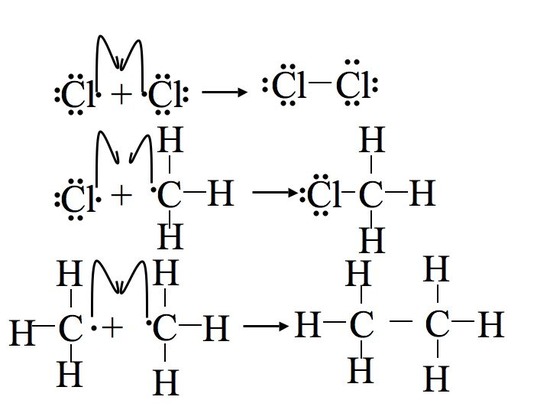
Limitations of the Chlorination
The chlorination of methane or any other alkane does not necessarily stop after one chlorination. It may actually be very hard to get a monosubstituted chloromethane. Instead di-, tri- and even tetra-chloromethanes are formed. One way to avoid this problem is to use a much higher concentration of methane or other alkane in comparison to chloride. This reduces the chance of a chlorine radical running into a chloromethane and starting the mechanism over again to form a dichloromethane. Through this method of controlling product ratios one is able to have a relative amount of control over the product.
Exercises
- Compounds other than chlorine and methane can react via free-radical halogenation. Write out the complete mechanism for the monobromination of ethane.
- Explain how the energetically unfavorable first propagation step can continue to occur without the input of energy from an external source.
- Which step of the radical chain mechanism requires outside energy? What can be used as this energy?
- Use the table provided below to calculate the change in enthalpy for the monobromination of ethane.
| Compound | Bond Dissociation Energy (kcal/mol) |
| CH3CH2-H | 101 |
| CH3CH2-Br | 70 |
| H-Br | 87 |
| Br2 | 46 |
Solutions
1.
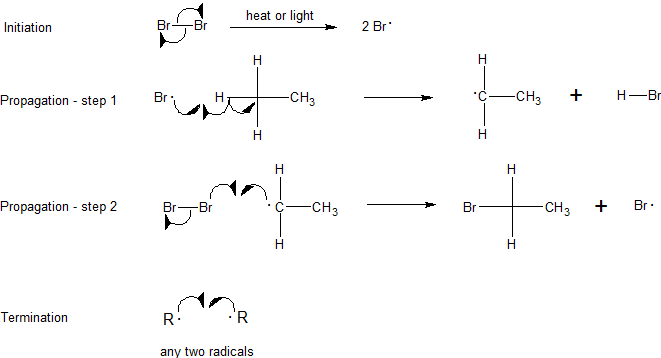
2. The exothermic energy released from the second propagation step provides the activation energy for the first propagation step creating a cyclic chain reaction following Le Chatelier's principle until termination.
3. The initiation step requires energy from heat o lit. For maximum photoefficiency, the wavelength of light is correlated with bond being homolytically cleaved.
4. 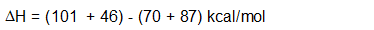
References
- Matyjaszewski, Krzysztof, Wojciech Jakubowski, Ke Min, Wei Tang, Jinyu Huang, Wade A. Braunecker, and Nicolay V. Tsarevsky. "Diminishing Catalyst Concentration in Atom Transfer Radical Polymerization with Reducing Agents." Science 72 (1930): 379-90.
- Phillips, Francis C. "# Researches upon the Chemical Properties of Gases." Researches upon the Chemical Properties of Gases 17 (1893): 149-236.
Outside Links
- Video of Mechanism: http://www.jbpub.com/organic-online/movies/chlormet.htm
- Wikipedia of Radical Chain Mechanism: en.Wikipedia.org/wiki/Free_radical_halogenation
- Wikipedia of Le Chatelier's Principle: en.Wikipedia.org/wiki/Le_Chatelier%27s_principle#Concentration
Contributors and Attributions
- Kristen Kelley and Britt Farquharson

