Liquid Chromatography
- Page ID
- 202041
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Liquid chromatography is a technique used to separate a sample into its individual parts. This separation occurs based on the interactions of the sample with the mobile and stationary phases. Because there are many stationary/mobile phase combinations that can be employed when separating a mixture, there are several different types of chromatography that are classified based on the physical states of those phases. Liquid-solid column chromatography, the most popular chromatography technique and the one discussed here, features a liquid mobile phase which slowly filters down through the solid stationary phase, bringing the separated components with it.
General Scheme
Components within a mixture are separated in a column based on each component's affinity for the mobile phase. So, if the components are of different polarities and a mobile phase of a distinct polarity is passed through the column, one component will migrate through the column faster than the other. Because molecules of the same compound will generally move in groups, the compounds are separated into distinct bands within the column. If the components being separated are colored, their corresponding bands can be seen. Otherwise as in high performance liquid chromatography (HPLC), the presence of the bands are detected using other instrumental analysis techniques such as UV-VIS spectroscopy1. The following figure shows the migration of two components within a mixture:
In the first step, the mixture of components sits atop the wet column. As the mobile phase passes through the column, the two components begin to separate into bands. In this example, the red component has a stronger affinity for the mobile phase while the blue component remains relatively fixed in the stationary phase. As each component is eluted from the column, each can be collected separately and analyzed by whatever method is favored. The relative polarities of these two compounds are determined based on the polarities of the stationary and mobile phases. If this experiment were done as normal phase chromatography, the red component would be less polar than the blue component. On the other hand, this result yielded from reverse phase chromatography would show that the red component is more polar than the blue component.
History of Liquid Chromatography
The first known chromatography is traditionally attributed to Russian botanist Mikhail Tswett who used columns of calcium carbonate to separate plant compounds during his research of chlorophyll. This happened in the 20th century (1901). Further development of chromatography occurred when the Nobel Prize was awarded to Archer John Porter Martin and Richard Laurence Millington Synge in 1952. They were able to establish the basics of partition chromatography and also develop Plate theory.
Column Chromatography
The stationary phase in column chromatography is most typically a fine adsorbent solid; a solid that is able hold onto gas or liquid particles on its outer surface. The column typically used in column chromatography looks similar to a Pasteur pipette (Pasteur pipettes are used as columns in small scale column chromatography). The narrow exit of the column is first plugged with glass wool or a porous plate in order to support the column packing material and keep it from escaping the tube. Then the adsorbent solid (usually silica) is tightly packed into the glass tube to make the separating column. The packing of the stationary phase into the glass column must be done carefully to create a uniform distribution of material. A uniform distribution of adsorbent is important to minimize the presence of air bubbles and/or channels within the column. To finish preparing the column, the solvent to be used as the mobile phase is passed through the dry column. Then the column is said to be "wetted" and the column must remain wet throughout the entire experiment. Once the column is correctly prepared, the sample to be separated is placed at the top of the wet column. A photo of a packed separating column can be found in the links.
Components
Chromatography is effective because different components within a mixture are attracted to the adsorbent surface of the stationary phase with varying degrees depending on each components polarity and its unique structural characteristics, and also its interaction with the mobile phase. The separation that is achieved using column chromatography is based on factors that are associated with the sample. So, a component that is more attracted to the stationary phase will migrate down the separating column at a slower rate than a component that has a higher affinity for the mobile phase. Also, the efficacy of the separation is dependent on the nature of the adsorbent solid used and the polarity of the mobile phase solvent.
Stationary Phase
The type of adsorbent material used as the stationary phase is vital for efficient separation of components in a mixture. Several different solid may be employed. Adsorbent material can be chosen based on particle size and activity of the solid. The activity of the adsorbent is represented by its activity grade, which is a measure of an adsorbent's attraction for solutes in the sample solution. The solids with the highest activity grading are those that are completely anhydrous. Silica gel and alumina are among the most popular adsorbents used. Alumina caters well to samples that that require specific conditions to adequately separate. However, the use of non-neutral stationary phases should be done with great caution, an increase or decrease of pH in the alumina stationary phase may allow chemical reactions within the components of the mixture. Silica gel, however, is less active than alumina and can generally be used as an all-around adsorbent for most components in solution. Silica is also preferred because of its high sample capacity, making it one of the most popular adsorbent materials.
Mobile Phase
The proper mobile phase must also be chosen for the best separation of the components in an unknown mixture. This eluent will be chosen based on its polarity relative to the sample and the stationary phase. With a strong polar adsorbent stationary phase like alumina, a polar solvent used as the mobile phase will be adsorbed by the stationary phase, which may displace molecules of sample in the mixture and may cause the sample components to elute vary quickly. This will provide little separation of the sample, so it is best to start elution with a solvent of lower polarity to elute the components that are weakly adsorbed to the stationary phase first. The solvent may also be changed during separation in order to change the polarity and therefore elute the various components separately in a more timely manner. This method is very similar to the gradient method of separation used in High Performance Liquid Chromatography (HPLC).
Types of Chromatography
- Normal Phase Chromatography: The components in a mixture will elute at different rates depending on each one's polarity relative to the next. When the column to be used for the separation is more polar than the mobile phase, the experiment is said to be a normal phase method. In normal phase chromatography, the stationary phase is polar, and so the more polar solutes being separated will adhere more to the stationary adsorbent phase. When the solvent or gradient of solvents is passed through the column, the less polar components will be eluted faster than the more polar ones. The components can then be collected separately, assuming adequate separation was achieved, in order of increasing polarity. This method of chromatography is not unique to liquid-solid column chromatography and is often used when performing High Performance Liquid Chromatography (HPLC). Although HPLC is an example of liquid-liquid chromatography, in which both the stationary and mobile phases are liquid, normal phase elution is achieved by coating the solid adsorbent column with a polar liquid.
- Reverse Phase Chromatography: In reverse phase chromatography, the polarities of the mobile and stationary phases are opposite to what they were when performing normal phase chromatography. Instead of choosing a non-polar mobile phase solvent, a polar solvent wil be chosen. Or, if the experiment requires a solvent polarity gradient, the gradient must be carried out with the most polar solvent first and the least polar solvent last (reverse order of normal phase chromatography). Common polar solvents mixtures of solvents include water, methanol, and acetonitrile. It is slightly more difficult and expensive to obtain a column where the stationary phase is non polar, as all solid adsorbents are polar by nature. The non polar stationary phase can be prepared by coating silanized silica gel with a non polar liquid. Silanizing the silica gel reduces the silica gel's ability to adsorb polar molecules. Common non polar liquid phases include silicone and various hydrocarbons. An alternative to this type of column is used in HPLC, in which a bonded liquid phase is used as the stationary phase. The less polar liquid is chemically bonded to the polar silica gel in the column. So using reverse phase, the most polar compounds in the sample solution will be eluted first, with the components following having decreasing polarities.
- Flash Chromatography: Because the elution rate of the mobile phase in regular column chromatography as described above is controlled primarily by gravity, chromatographic runs can potentially take a very long time to complete. Flash chromatography is a modified method of column chromatography in which the mobile phase moves faster through the column with the help of either pressurized air or a vacuum. A vacuum line is attached to the bottom of the separating column, this pulls the mobile phase solvent, and the components in the mobile phase, through the column at a faster rate than gravity does. A figure of this set-up can be seen in the links section. Flash chromatography is powered by compressed air or air pumps works by pushing the mobile phase through the column and achieves faster flow rates of the mobile phase just as vacuum facilitated flash chromatography does. For this method, a pressurized air line is attached to the top of the separating column. It is for this reason that flash chromatography is also referred to as medium pressure chromatography. An inert gas is used as to not interact with the mobile or stationary phase or the component mixture. Nitrogen gas is commonly used for this method of chromatography. Many instruments are available to perform flash chromatography as efficiently as possible: expensive columns, pumps, and flow controllers. This maintains a constant and precise air pressure or vacuum to the column in order to obtain steady flow rate of the mobile phase and favorable separation of the samples in solution. However, less expensive alternatives are available, as flow controllers can be made so that pressurized air can be used to facilitate flash chromatography.
By using the above apparatus, purchasing expensive air pumps can be avoided. This method is useful to an extent. Since the flow rate of the pressurized gas is controlled manually by the flow rate controller, it is more difficult to quantify the flow rate and keep that flow rate constant. Instruments available for flash chromatography are able to set flow rates digitally and keep flow rate constant.
Flash chromatography is similar to HPLC in that the mobile phase is moved through the column by applying pressure to the solvent in order to achieve a quicker result. However, in flash chromatography, only medium pressure is applied to the system within the solution. In HPLC, pressures as high as 5000 psi can be applied in the column by high performance pumps.
Other Varieties of Liquid Chromatography
- Partition Chromatography: In this method, both the stationary phase and the mobile phase are liquid. The stationary phase liquid would be an immiscible liquid with the mobile phase.
- Liquid-Solid Chromatography: This method is similar to partition chromatography only that the stationary phase has been replaced with a bonded rigid silica or silica based component onto the inside of the column. Sometimes the stationary phase may be alumina. The analytes that are in the mobile phase that have an affinity for the stationary phase will be adsorbed onto it and those that do not will pass through having shorter retention times. Both normal and reverse phases of this method are applicable.
- Ion Exchange or Ion Chromatography: This is a type of chromatography that is applied to separate and determine ions on columns that have a low ion exchange capacity. This is based on the equilibrium of ion exchange between the ions in solution and the counter ions to pair with the oppositely charged ions that are fixed to the stationary phase. This stationary phase would either have positive of negative functional groups affixed to it, usually sulfonate (-SO3-) or a quaternary amine (-N(CH3)3+), being a cation and anion exchanger respectively.
- Size Exclusion Chromatography: Size exclusion chromatography separates molecules by their size. This is done by having the stationary phase be packed with small particles of silica or polymer to form uniform pores. The smaller molecules will get trapped in the silica particles and will elude from the column at a rate that is greater than that of larger molecules. Thus, the retention time depends on the size of the molecules. Larger molecules will be swept away in the mobile phase, therefore having a smaller retention time. Also notice that in this type of chromatography there isn’t any interaction, being physical or chemical, between the analyte and the stationary phase.
- Affinity Chromatography: This type of chromatography involves binding a reagent to the analyte molecules in a sample. After the binding, only the molecules that have this ligand are retained in the column, the unbound analyte is passed through in the mobile phase. The stationary phase is usually agrose or a porous glass bead that is able to immobilize the bonded molecule. It is possible to change the elution conditions by manipulating the pH or the ionic strength of the binding ligand. This method is often used in biochemistry in the purification of proteins. The ligand tag is bonded and after separation the tag is then removed and the and the pure protein is obtained.
- Chiral Chromatography: Chiral chromatography enables the use of liquid chromatography to separate a racemic mixture into its enantiomeric parts. A chiral additive can be added to the mobile phase, or a stationary phase that has chiral properties can be used. A chiral stationary phase is the most popular option. The stationary phase has to be chiral in order to recognize the chirality of the analyte, this will create attractive forces between the bonds and also form inclusion complexes.
Plate Theory and Rate Theory
Plate theory and Rate theory are two theories that are applicable to chromatography. Plate theory describes a chromatography system as being in equilibrium between the stationary and mobile phases. This views the column as divided into a number of imaginary theoretical plates. This is significant because as the number of plates in a column increases or the height equivalent theoretical plates or HETP increases, so does the separation of components. It also provides an equation that describes the elution curve or the chromatogram of a solute it can also be used to find the volume and the column efficiency.
\[HETP = \dfrac{L}{N} \]
where L= column length and N= number of theoretical plates
The Rate theory on the other hand describes the migration of molecules in a column. This included band shape, broadening, and the diffusion of a solute. Rate theory follows the Van Deemter equation, which is the most appropriate for prediction of dispersion in liquid chromatography columns. It does this by taking into account the various pathways that a sample must travel through a column. Using the Van Deemter equation, it is possible to find the optimum velocity and and a minimum plate height.
\[ H=A+\dfrac{B}{u} = Cu \]
where \(A\) = Eddy-Diffusion, \(B\) = Longitudinal Diffusion, \(C\) = mass transfer, \(u\) = linear velocity
Instrumentation
This schematic is of the basic instrumentation of a liquid-solid chromatograph. The solvent inlet brings in the mobile phase which is then pumped through the inline solvent filter and passed through the injection valve. This is where the mobile phase will mix with the injected sample. It then gets passed through another filter and then passed through the column where the sample will be separated into its components. The detector detects the separation of the analytes and the recorder, or usually a computer will record this information. The sample then goes through a backpressure filter and into waste.
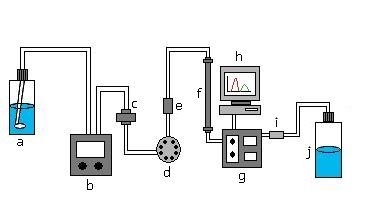
A basic LC system consists of (a) a solvent inlet filter, (b) pump, (c) inline solvent filter, (d) injection valve, (e) precolumn filter, (f) column, (g) detector, (h) recorder, (i) backpressure regulator, and a (j) waste reservoir.
Advantages / Disadvantages
Liquid-solid column chromatography is an effective separation technique when all appropriate parameters and equipment are used. This method is especially effective when the compounds within the mixture are colored, as this gives the scientist the ability to see the separation of the bands for the components in the sample solution. Even if the bands are not visible, certain components can be observed by other visualization methods. One method that may work for some compounds is irradiation with ultraviolet light. This makes it relatively easy to collect samples one after another. However, if the components within the solution are not visible by any of these methods, it can be difficult to determine the efficacy of the separation that was performed. In this case, separate collections from the column are taken at specified time intervals. Since the human eye is the primary detector for this procedure, it is most effective when the bands of the distinct compounds are visible.
Liquid-solid column chromatography is also a less expensive procedure than other methods of separation (HPLC, GC, etc.). This is because the most basic forms of column chromatography do not require the help of expensive machinery like high pressure solvent pumps used in HPLC. In methods besides flash chromatography, the flow of the mobile phase, the detection of each separation band, and the collection of each component, are all done manually by the scientist. Although this introduces many potential instances of experimental error, this method of separation can be very effective when done correctly. Also, the glass wear used for liquid-solid column chromatography is relatively inexpensive and readily available in many laboratories. Burets are commonly used as the separating column, which in many cases will work just as well as an expensive pre-prepared column. For smaller scale chromatography, Pasteur pipettes are often used.
Flash chromatography has the potential to be more costly than the previous methods of separation, especially when sophisticated air pumps and vacuum pumps are needed. When these pieces of machinery are not needed, however, a vacuum line can be instead connected to an aspirator2 on a water faucet. Also, home-made pressurized air flow controllers can be made as shown previously.
References
- Jones Jr., M. Organic Chemistry, 2nd ed. New York, NY. W. W. Norton & Company, Inc. 2000.
- Lehman, JW. Operational Organic Chemistry, 3rd ed. Upper Saddle River, NJ. Prentice Hall. 2002.
- Skoog, DA; Holler, FJ; Crouch, SR. Principles of Instrumental Analysis, 6th ed. Belmont, CA. Thomson Higher Education. 2007.
- Wade Jr., LG. Organic Chemistry, 6th ed. Upper Saddle River, NJ. Prentice Hall. 2006.
Contributors
- Jennifer Betancourt (UC Davis), Sean Gottlieb (UC Davis)

