3: Chemical Nomenclature (Experiment)
- Page ID
- 221124
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Nomenclature of Ionic Compounds
Ionic compounds are composed of ions. An ion is an atom or molecule with an electrical charge. Monatomic ions are formed from single atoms that have gained or lost electrons. Polyatomic ions are formed from molecules (groups of atoms bonded together) that have gained or lost electrons.
Negative ions are called anions, and are formed when an atom or molecule gains electrons. All non- metals form negatively charged ions. Positive ions are called cations, and are formed when an atom or molecule loses electrons. All metals form positively charged cations. Ions with opposite charges (positive metal cations and negative non-metal anions) will experience a strong electrostatic attraction and form an ionic bond, which leads to the formation of the ionic compound.
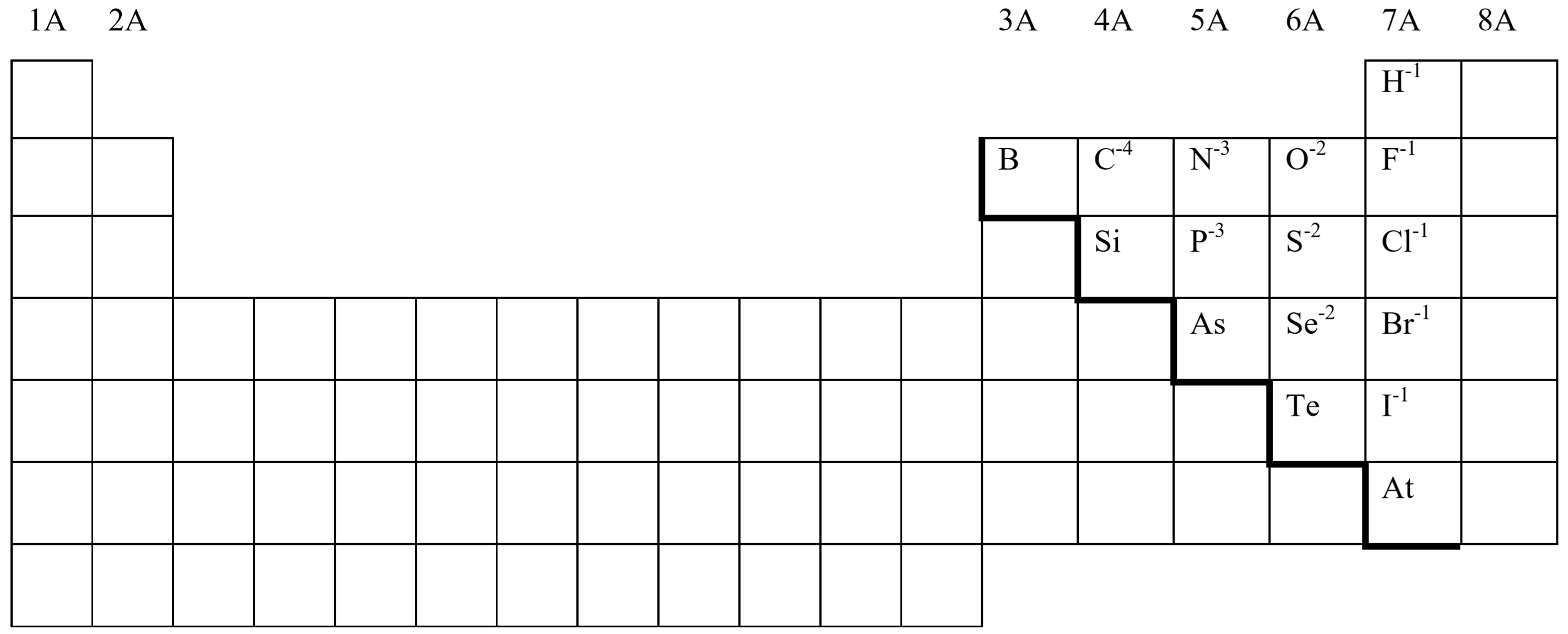
Non-metal Anions
Non-metals will form anions with only one possible negative charge. The following Periodic Table shows the charges for non-metal anions commonly found in ionic compounds:
Note that
- The magnitude of the negative charges on these anions is equal to 8 minus their Group Number.
- The names of these anions are based on the element names, but the endings are all changed to –ide.
Example \(\PageIndex{1}\):
Sulfur in Group 6A forms anions with a \([8–6] = 2\), or, –2 charge.
Example \(\PageIndex{2}\):
The \(\ce{Cl^{-1}}\) anion is called the chloride ion.
Metal Cations
Most (but not all) main group metals will form cations with only one possible charge. Most (but not all) transition metals will form cations with more than one possible charge. The following Periodic Table shows the charges for metal cations commonly found in ionic compounds:
Note that
- The magnitude of the positive charge on the main group metal cations is generally equal to their Group Number.
- The names of metal cations with only one possible charge are the same as the names of the metals themselves.
- For metal cations with more than one possible charge, the ion charge must be indicated in the ion name. In the IUPAC system, the ion charge is indicated in the name as Roman numerals in brackets.
Example \(\PageIndex{3}\):
Magnesium in Group 2A forms cations with a +2 charge.
Example \(\PageIndex{4}\):
\(\ce{Al^{+3}}\) is called the aluminum cation.
\(\ce{Ag^{+1}}\) is called the silver cation.
Example \(\PageIndex{5}\):
\(\ce{Pb^{+2}}\) is called the lead(II) cation.
\(\ce{Pb^{+4}}\) is called the lead(IV) cation.
Polyatomic Ions
Polyatomic ions are formed from molecules (groups of atoms bonded together) that have gained or lost electrons. The table below includes a list of common polyatomic ions that must be memorized.
|
\(\ce{OH^{-1}}\) |
Hydroxide |
\(\ce{O2^{-2}}\) |
Peroxide |
|
\(\ce{CN^{-1}}\) |
Cyanide |
\(\ce{CO3^{-2}}\) |
Carbonate |
|
\(\ce{SCN^{-1}}\) |
Thiocyanate |
\(\ce{SO3^{-2}}\) |
Sulfite |
|
\(\ce{HCO3^{-1}}\) |
Bicarbonate (Hydrogen Carbonate) |
\(\ce{SO4^{-2}}\) |
Sulfate |
|
\(\ce{HSO3^{-1}}\) |
Bisulfite (Hydrogen Sulfite) |
\(\ce{S2O3^{-2}}\) |
Thiosulfate |
|
\(\ce{HSO4^{-1}}\) |
Bisulfate (Hydrogen Sulfate) |
\(\ce{C2O4^{-2}}\) |
Oxalate |
|
\(\ce{C2H3O2^{-1}}\) |
Acetate |
\(\ce{CrO4^{-2}}\) |
Chromate |
|
\(\ce{NO2^{-1}}\) |
Nitrite |
\(\ce{Cr2O7^{-2}}\) |
Dichromate |
|
\(\ce{NO3^{-1}}\) |
Nitrate | ||
|
\(\ce{MnO4^{-1}}\) |
Permanganate |
\(\ce{PO3^{-3}}\) |
Phosphite |
|
\(\ce{ClO^{-1}}\) |
Hypochlorite |
\(\ce{PO4^{-3}}\) |
Phosphate |
|
\(\ce{ClO2^{-1}}\) |
Chlorite | ||
|
\(\ce{ClO3^{-1}}\) |
Chlorate |
\(\ce{NH4^{+1}}\) |
Ammonium |
|
\(\ce{ClO4^{-1}}\) |
Perchlorate |
\(\ce{Hg2^{+2}}\) |
Mercury (I) |
Note that
- Almost all the polyatomic ions are negatively charged anions.
- Most of the names of polyatomic anions end in either –ate or –ite. The –ate’s always have one more oxygen than the -ite’s.
Example \(\PageIndex{6}\):
Sulfate, \(\ce{SO4^{-2}}\), has one more oxygen atom present than sulfite, \(\ce{SO3^{-2}}\).
Formulas and Names of Ionic Compounds
Ionic compounds are formed when positive cations and negative anions are attracted to each other via strong electrostatic forces. This attraction is called an ionic bond. The following are the basic rules for writing the formulas and names of ionic compounds:
Writing Formulas
- Determine the formulas and charges on the cation and anion involved in the compound.
- Combine the ions in a ratio that results in the formation of a neutral ionic compound. In other words, the total charge of all the positive cations must equal the total charge of all the negative anions in the compound. The numbers of each element present in the compound are shown as subscripts after the element symbol.
Writing Names
- Both the cation and anion must be named.
- Always name the cation first, then the anion.
Example \(\PageIndex{7}\):
Write the formula and name for the compound formed between calcium and fluorine
\(\ce{Ca}\) (metal) forms a +2 cation \(\ce{Ca^{+2}}\), the calcium cation.
\(\ce{F}\) (non-metal) forms a -1 anion \(\ce{F^{-1}}\), the fluoride anion.
To obtain a neutral compound, 1 \(\ce{Ca^{+2}}\) is needed for every 2 \(\ce{F^{-1}}\)
The formula of the compound is \(\ce{CaF2}\)
The name of the compound is Calcium Fluoride
Example \(\PageIndex{8}\):
Write the formula for iron(III) chloride.
First identify the cation and the anion in this compound.
Cation = iron(III) = \(\ce{Fe^{+3}}\) (transition metal cation)
Anion = chloride = \(\ce{Cl^{-1}}\) (non-metal anion)
To obtain a neutral compound, 1 \(\ce{Fe^{+3}}\) is needed for every 3 \(\ce{Cl^{-1}}\)
The formula of the compound is \(\ce{FeCl3}\)
Example \(\PageIndex{9}\):
Write the formula for magnesium phosphate.
First identify the cation and anion in this compound.
Cation = magnesium = \(\ce{Mg^{+2}}\) (metal cation)
Anion = phosphate = \(\ce{PO4^{-3}}\) (polyatomic anion)
To obtain a neutral compound, 3 \(\ce{Mg^{+2}}\) are needed for every 2 \(\ce{PO4^{-3}}\)
The formula of the compound is \(\ce{Mg3(PO4)2}\)
Note in the above Example 9 that parentheses are placed around the polyatomic portion of compound, to indicate that it must be treated as a complete and whole unit.
Example \(\PageIndex{10}\):
Name the ionic compound \(\ce{Al(NO3)3}\).
First identify the cation and anion in this compound.
Cation = \(\ce{Al^{+3}}\) = the aluminum cation (metal cation)
Anion = \(\ce{NO3^{-1}}\) = the nitrate anion (polyatomic anion)
The name of this compound is Aluminum Nitrate
Example \(\PageIndex{11}\):
Name the compound \(\ce{TiO2}\).
First identify the cation and anion in this compound.
Cation = \(\ce{Ti^{+4}}\) = the titanium(IV) cation (transition metal cation)
Anion = \(\ce{O^{-2}}\) = the oxide anion (non-metal anion)
The name of the compound is Titanium(IV) Oxide
Nomenclature of Simple Covalent Compounds
Covalent compounds are compounds formed between non-metals only. Simple binary covalent compounds contain just two different types of non-metal elements. When non-metals combine they can form several different covalent compounds. These compounds must therefore be identified with unique names and formulas.
Example \(\PageIndex{12}\):
Carbon and oxygen combine to form two common covalent compounds \(\ce{CO2}\) and \(\ce{CO}\).
Formulas and Names of Simple Covalent Compounds
- Always write/name the element with more metallic character first. Metallic character increases going from right to left, and top to bottom on the Periodic Table.
- Then write/name the second (less metallic) element, changing the ending of its name to -ide.
- Since nonmetals often combine in different proportions to form a number of different compounds, prefixes must be included in the names to indicate the numbers of each kind of atom present.Prefixes for 1-10 atoms are given in the following table.
|
Number |
Prefix | Number |
Prefix |
|---|---|---|---|
|
1 |
Mono | 6 |
Hexa |
|
2 |
Di | 7 |
Hepta |
|
3 |
Tri | 8 |
Octa |
|
4 |
Tetra | 9 |
Nona |
|
5 |
Penta | 10 |
Deca |
Example \(\PageIndex{13}\):
A compound contains 3 atoms of sulfur and 4 atoms of phosphorus. Write its name and formula.
Since phosphorus is the more metallic element (left of sulfur), it must be named first.
Sulfur being the less metallic element is named second with an -ide ending = sulfide.
The prefix for three is tri, and the prefix for four is tetra.
The name of this compound is tetraphosphorus trisulfide
The formula of this compound is \(\ce{P4S3}\)
Example \(\PageIndex{14}\):
Write the name of the compound \(\ce{N2O}\).
The two N atoms require the prefix di in the name.
The one O atom requires the prefix mono in the name. The ending of oxygen must be changed to –ide = oxide.
The name of this compound is dinitrogen monoxide
There are two important exceptions to the naming rules outlined so far:
- Never use the prefix “mono” for the first element, even if just one atom is present.
- Never use any prefixes at all for simple covalent compounds containing Hydrogen.
Example \(\PageIndex{15}\):
Write the name of the compound \(\ce{BCl3}\).
Although just one B atom is present, the prefix mono is not used since it is the first element in this compound.
The three Cl atoms require the prefix tri in the name. The ending of chlorine must be changed to –ide = chloride.
The name of this compound is boron trichloride
Example \(\PageIndex{16}\):
\(\ce{HF}\) is hydrogen fluoride not hydrogen monofluoride or monohydrogen monofluoride.
Please note that many simple covalent compounds have common, rather than systematic names. Please memorize the common names of the following three compounds:
- \(\ce{H2O}\) water
- \(\ce{NH3}\) ammonia
- \(\ce{CH4}\) methane
Covalent compounds containing more than two non-metal elements become increasingly more difficult to name, and common names for these compounds are more extensively used. You will not have to learn these yet.
Nomenclature of Acids
Acids are compounds that release hydrogen cations (\(\ce{H^{+1}}\)) when dissolved in water. They are all found in the aqueous state (aq).
\[\ce{HCl (aq) -> H^{+1} (aq) + Cl^{-1} (aq)}\]
\[\text{hydrochloric acid} \ce{->}\text{ hydrogen ions} + \text{chloride ions}\]
\[\ce{HNO3 (aq) -> H^{+1} (aq) + NO3^{-1} (aq)}\]
\[\text{nitric acid} \ce{->} \text{hydrogen ions} + \text{nitrate ions}\]
\[\ce{H2SO3 (aq) <-> 2 H^{+1} (aq) + SO3^{-2} (aq)}\]
\[\text{sulfurous acid} \ce{<->} \text{hydrogen ions} + \text{sulfite ions}\]
In acids the element hydrogen actually behaves like a Group 1A metal cation. Since it behaves like a +1 cation, hydrogen is always written first in the formulas of all acids. The anion in the acid can be either monatomic or polyatomic, and affects how the acid is named.
Acids containing Non-Metal Anions
These acids contain the \(\ce{H^{+1}}\) cation and a monatomic non-metal anion.
Example \(\PageIndex{17}\):
Hydrochloric acid, \(\ce{HCl}\) (aq), contains \(\ce{H^{+1}}\) and the monatomic anion, chloride, \(\ce{Cl^{-1}}\).
Acids containing monatomic anions are named using the prefix hydro + the name of the anion with the suffix -ic + the word acid.
Example \(\PageIndex{18}\):
Name the acid \(\ce{HBr}\) (aq).
This acid contains \(\ce{H^{+1}}\) and the monatomic anion, bromide, \(\ce{Br^{-1}}\).
The name of this acid is hydro + brom-ic + acid = hydrobromic acid
The formulas of these acids are obtained in an identical fashion to regular ionic compounds. The \(\ce{H^{+1}}\) cation and the monatomic anion are combined in a ratio to yield a neutral compound.
Example \(\PageIndex{19}\):
Write the formula and name for the acid containing the phosphide anion.
This acid contains \(\ce{H^{+1}}\) and the \(\ce{P^{-3}}\) anion (monatomic).
Just combine them together like any other cation and anion: 3 \(\ce{H^{+1}}\) : 1 \(\ce{P^{-3}}\)
The formula of this acid is \(\ce{H3P}\) (aq)
\(\ce{H3P}\) (aq) is named hydro + phosphor-ic + acid = hydrophosphoric acid
Acids containing Polyatomic Anions
These acids contain the \(\ce{H^{+1}}\) cation and a polyatomic anion.
Example \(\PageIndex{20}\):
Nitric acid, \(\ce{HNO3}\) (aq), contains \(\ce{H^{+1}}\) and the polyatomic anion, nitrate, \(\ce{NO3^{-1}}\).
These acids have names that are based on the name of the polyatomic ion in the acid. If the polyatomic ion has the ending -ate, in the acid the ending is changed to -ic + acid. If the polyatomic ion has the ending -ite, in the acid the ending is changed to -ous + acid.
Example \(\PageIndex{21}\):
Name the acid \(\ce{HClO3}\) (aq).
This acid contains \(\ce{H^{+1}}\) and the polyatomic anion \(\ce{ClO3^{-1}}\) = chlorate.
To name this acid, the ending -ate is switched to -ic.
The acid \(\ce{HClO3}\) (aq) is thus called chloric acid
Example \(\PageIndex{22}\):
Name the acid \(\ce{H2SO3}\) (aq).
This acid contains \(\ce{H^{+1}}\) and the polyatomic anion \(\ce{SO3^{-2}}\) = sulfite.
To name this acid, the ending -ite is switched to -ous.
The acid \(\ce{H2SO3}\) (aq) is thus named sulfurous acid
Again, the formulas of these acids are obtained in an identical fashion to regular ionic compounds. The \(\ce{H^{+1}}\) cation and the polyatomic anion are combined in a ratio to yield a neutral compound.
Example \(\PageIndex{23}\):
Write the formula for oxalic acid.
Oxalic acid must contain (by reverse logic) the polyatomic anion oxalate = \(\ce{C2O4^{-2}}\), as well as \(\ce{H^{+1}}\) (the cation in all acids).
Once the ions have been identified, just combine them together like any other cation and anion: 2 \(\ce{H^{+1}}\) : 1 \(\ce{C2O4^{-2}}\)
The formula of oxalic acid is \(\ce{H2C2O4}\) (aq)
Nomenclature of Hydrates
A hydrate is typically an ionic compound with a certain number of water molecules loosely bound to it.
The general formula of a hydrate is \(\ce{MX*nH2O}\) (s), where \(\ce{M}\) is the cation in the ionic compound, \(\ce{X}\) is the anion in the ionic compound and \(\ce{nH2O}\) are the water molecules loosely bound to the ionic compound.
Hydrates are named by writing the name of the ionic compound first, followed by the word “hydrate”. To indicate the number of water molecules present, prefixes must be used.
Example \(\PageIndex{24}\):
Name the hydrate \(\ce{MgSO4*7H2O}\).
\(\ce{MgSO4}\) is the ionic compound magnesium sulfate.
Since there are seven water molecules present, the correct prefix to use is hepta.
The name of this hydrate is magnesium sulfate heptahydrate
Example \(\PageIndex{25}\):
Write the formula for copper(II) chloride dihydrate.
Copper(II) chloride has the formula \(\ce{CuCl2}\)
The prefix di indicates that there are two water molecules present.
The formula of this hydrate is \(\ce{CuCl2*2H2O}\)
The water molecules in a hydrate can be removed with relative ease by heating the hydrate. The ionic compound that remains after heating is called an anhydrous salt.
\[\ce{MX*nH2O (s) -> MX (s) + n H2O}\]
\[\text{Hydrate} \ce{->} \text{Anhydrous salt} + \text{Free Water}\]
Often the anhydrous salt has a completely different color and texture from the hydrate.
Example \(\PageIndex{26}\):
Copper(II) sulfate pentahydrate is blue and crystalline, whereas anhydrous copper(II) sulfate is white and powdery
Lab Report: Chemical Nomenclature
Write the names and formulas for the following inorganic compounds in the spaces provided.
Part 1: Ions and Ionic Compounds
Write formulas/charges or names as appropriate for each of the following monatomic ions.
- Calcium ion
- Phosphide ion
- Iodide ion
- Gallium ion
- Titanium(IV) ion
- \(\ce{C^{-4}}\)
- \(\ce{Rb^{+1}}\)
- \(\ce{Pb^{+4}}\)
- \(\ce{S^{-2}}\)
- \(\ce{Cr^{+2}}\)
Write formulas or names as appropriate for each of the following ionic compounds.
- Magnesium nitride
- Lithium oxide
- Aluminum sulfite
- Copper(II) bicarbonate
- Sodium nitrate
- \(\ce{SrI2}\)
- \(\ce{Ba3(PO4)2}\)
- \(\ce{(NH4)2O}\)
- \(\ce{Fe(ClO)3}\)
- \(\ce{ZnCrO4}\)
Part 2: Covalent Compounds
Write formulas or names as appropriate for each of the following covalent compounds.
- Dichlorine monoxide
- Disulfur dichloride
- Carbon tetrafluoride
- Phosphorus pentachloride
- Nitrogen tribromide
- \(\ce{AsI3}\)
- \(\ce{P4O10}\)
- \(\ce{Cl2O7}\)
- \(\ce{SeCl6}\)
- \(\ce{NO}\)
Part 3: Acids
Write formulas or names as appropriate for each of the following acids.
- Hydroiodic acid
- Carbonic acid
- Chlorous acid
- Sulfuric acid
- Phosphorous acid
- \(\ce{HCN}\) (aq)
- \(\ce{H2C2O4}\) (aq)
- \(\ce{HNO2}\) (aq)
- \(\ce{H2Cr2O7}\) (aq)
- \(\ce{HMnO4}\) (aq)
Part 4: Hydrates (optional – check with your instructor to see if you are responsible for this section)
Write formulas or names as appropriate for each of the following hydrates.
- Magnesium sulfate heptahydrate
- Copper(I) sulfate pentahydrate
- Potassium phosphate decahydrate
- Calcium chloride hexahydrate
- Iron(III) nitrate nonahydrate
- \(\ce{CoSO4*H2O}\)
- \(\ce{Na2CrO4*4H2O}\)
- \(\ce{CuF2*2H2O}\)
- \(\ce{Sr(NO3)2*6H2O}\)
- \(\ce{ZnSO4*7H2O}\)
Part 5: Nomenclature of Ionic Compounds, Covalent Compounds and Acids
|
Classification |
Name or Formula |
|
|---|---|---|
|
\(\ce{C3O2}\) |
||
|
\(\ce{IF7}\) |
||
|
\(\ce{Rb2CO3}\) |
||
|
\(\ce{SnS2}\) |
||
|
\(\ce{Au(CN)3}\) |
||
|
\(\ce{H2CrO4}\) (aq) |
||
|
\(\ce{H3P}\) (aq) |
||
|
\(\ce{Li3PO4}\) |
||
|
\(\ce{Mg3N2}\) |
||
|
\(\ce{Ti(C2H3O2)4}\) |
||
|
\(\ce{Fe2O3}\) |
||
|
\(\ce{NaH}\) |
||
|
\(\ce{Br3O8}\) |
||
|
\(\ce{MnS2O3}\) |
||
|
\(\ce{NH4NO2}\) |
||
|
\(\ce{Cd(ClO2)2}\) |
||
|
\(\ce{Ba(HSO3)2}\) |
||
|
\(\ce{Cu2O}\) |
||
|
\(\ce{NiBr3}\) |
||
|
\(\ce{Sr(OH)2}\) |
||
|
Perchloric acid |
||
|
Potassium permanganate |
||
|
Calcium hydride |
||
|
Vanadium(II) bicarbonate |
||
|
Bismuth(V) nitrate |
||
|
Rubidium peroxide |
| Strontium hydrogen phosphite | ||
|
Hydrofluoric acid |
||
|
Chromium(III) thiocyanate |
||
|
Acetic acid |
||
|
Molybdenum(IV) carbonate |
||
|
Tetraiodine nonaoxide |
||
|
Diphosphorus tetrafluoride |
||
|
Aluminum sulfate |
||
|
Ammonium hydroxide |
||
|
Sodium dichromate |
||
|
Carbon disulfide |
||
|
Nickel(II) oxalate |
||
|
Barium selenide |
||
|
Silver bisulfate |
Questions
- How are the following types of compounds recognized from their formulas?
- Ionic
- Covalent
- Acid
- When do parentheses appear in the formulas of ionic compounds?
- Do Roman Numerals appear in the names of ionic or covalent compounds? Explain why they are used.
- Do Greek Prefixes appear in the names of ionic or covalent compounds? Explain why they are used.
- What is the relationship between the number of hydrogens in an acid and the charge on the anion that they are combined with?