Thermite Reaction
- Page ID
- 222045
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
Chemical Concepts Demonstrated
- Exothermicity
- Heat capacity
- Oxidation/reduction
- Enthalpy/entropy
Demonstration
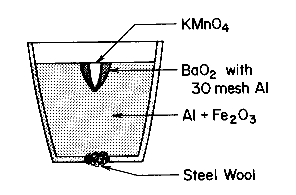
The crucible shown contains:
| The crucible is placed in a ring stand directly above the sand box. 3-5 drops of glycerin are added to the KMnO4 |
Observation
The reaction occurs, melts the steel wool at the bottom of the crucible, and causes liquid iron to pour out from the resulting gap.
Explanation (including important chemical equations)
This mechanism has an unfavorable entropy. A much more favorable enthalpy provokes and carries the reaction. The resulting heat from the demonstration itself melts the iron product formed. The total energy released in this experiment is equal to 851.5 kJ.
Fe2O3 + 2 Al -> 2 Fe + Al2O3
The term "Thermite" refers to the mixture of aluminum and ferric oxide used in this experiment. It is sold commercially and is used for such applications as railroad welding and incendiary bombs.

