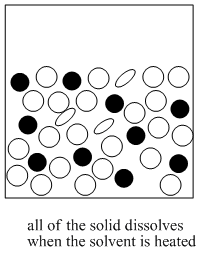
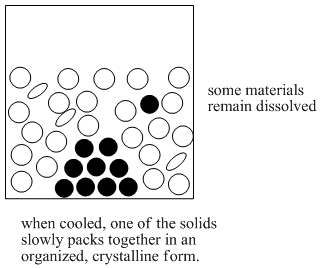
Recrystallization
- Page ID
- 221864
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Recrystallization is used to purify solids. Usually this method works best when there is only a small amount of impurity in the solid.
The method involves addition of a cold solvent to the material.
The mixture that results is heated until the solids dissolve.
The mixture is slowly cooled again until a pure solid is obtained.
Recrystallization depends on different solubilities of the target compound and other compounds present in the impure mixture. The goal of this method is to have one compound dissolved in a solvent while the other compound is not dissolved. If one compound is an undissolved solid, it can be filtered out of the solution in order to separate it from all the other things that are in solution.
Solubility in a solvent is a physical property of a material, just like its boiling point or melting point. Sodium chloride (table salt) has a particular solubility in cold water (35.7 g will dissolve in 100 mL) while sodium oleate (found in some soaps) has a different solubility in cold water (10 g per 100 mL). That difference can be exploited to separate these two compounds.
STOP AND THINK
1. Suppose you were trying to go into the soap business. Maybe you find you can easily produce a mixture of equal parts (weight:weight) of sodium chloride and sodium oleate. Describe how you could get pure sodium oleate.
2. Look at the structures of sodium chloride and sodium oleate. Can you qualitatively explain the difference between their solubilities in water?
It’s possible that a mixture of sodium chloride and sodium oleate could be purified through the addition of water. If the right amount of water were added and the resulting slurry were stirred together and filtered, much of the sodium chloride would be removed because it is more soluble in water than is sodium oleate. Sodium oleate is less soluble in water than is sodium chloride, so most of it would not dissolve. It could be gathered or “isolated” by filtration.
The technique described above is not recrystallization. It is referred to as “washing”. The sodium oleate was washed with water to remove sodium chloride. Washing is simple to do. Sometimes it can increase the purity of a compound, but it is not always very effective.
Sometimes in a mixture the two compounds are mixed very tightly together. Suppose there is a pile of powder that contains sodium oleate and sodium chloride. Instead of having individual grains of sodium chloride and individual grains of sodium oleate, the grains of powder contain both compounds. There might be a small nugget of sodium chloride surrounded by a coating of sodium oleate. This situation is very common, especially when the two compounds have formed together. Washing might remove most of the exposed sodium chloride, but it wouldn’t touch the hidden or “occluded” sodium chloride that was surrounded by sodium oleate molecules.
STOP AND THINK
3. How can you get all of the sodium chloride out of this mixture? In other words, how can you make sure there is no sodium chloride covered by sodium oleate?
It would be convenient if there were a switch that could be turned on and off to control dissolution. When the switch is on, all the sodium oleate dissolves, and any sodium chloride trapped inside can get dissolved, too. When the switch is off, the sodium oleate comes out of solution again so that you can filter it out.
This switch exists. It is found on the front of your hot plate in the organic lab. Solubility is temperature-dependent. For example, the solubility of sodium chloride is different in cold water (35.7 g per 100 mL at 0 C) than in hot water (39.12 g per 100 mL at 100 C).
It’s possible that, in the right amount of hot water, all the sodium oleate and all the sodium chloride dissolves. If the resulting solution is cooled to room temperature, the sodium oleate is no longer soluble. The sodium oleate forms a solid again. This solid can be filtered away from the water.
STOP AND THINK
4. After filtration, list the contents of the solution.
5. Instead of filtering, can the solution be allowed to evaporate to leave the sodium oleate behind?
There is one more part of this process that would make it a complete recrystallization. If the sodium oleate forms an amorphous solid when it comes back out of solution, we have precipitation. If the sodium oleate forms a crystalline solid when it comes back out of solution, we have recrystallization. An amorphous solid contains molecules that are packed together in random ways. A crystalline solid contains molecules that are ordered in a specific way.
An amorphous solid is like a pile of boxes that were thrown across the room and piled in the corner. A crystalline solid is like a set of boxes that were stacked neatly in the corner.
There are many open spaces between the boxes in the “amorphous” pile. Impurities could get trapped in those spaces; in that case we are back where we were at the beginning.
There are not really spaces between the boxes in the “crystalline” pile. No impurities can be trapped. Crystallization safeguards against the inclusion of impurities in the material.
STOP AND THINK
6. There are many variations on most purification techniques. One problem that could happen in a recrystallization is that there is a very insoluble impurity. How could this very insoluble impurity be separated from the rest of the sample? What precautions must be taken in doing so?
7. Suppose you are trying to recrystallize a sample of borneol. You think it should be recrystallized from methanol. You add a couple of mL of cold methanol and the sample all dissolves. Evaluate the prognosis of your recrystallization: is it working well? If not, what do you need to do?
8. Suppose you are trying to recrystallize a sample of borneol. You think it should be recrystallized from methanol. You add a couple of mL of cold methanol and only about half the sample dissolves. Evaluate the prognosis of your recrystallization: is it working well? If not, what do you need to do?
9. Suppose you are trying to recrystallize a sample of borneol. You think it should be recrystallized from methanol. You add a couple of mL of hot methanol and only about half the sample dissolves. Evaluate the prognosis of your recrystallization: is it working well? If not, what do you need to do?