7.E: Stoichiometry of Chemical Reactions (Exercises)
- Page ID
- 221180
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)4.1: Writing and Balancing Chemical Equations
Q4.1.1
What does it mean to say an equation is balanced? Why is it important for an equation to be balanced?
S4.1.1
An equation is balanced when the same number of each element is represented on the reactant and product sides. Equations must be balanced to accurately reflect the law of conservation of matter.
Q4.1.2
Consider molecular, complete ionic, and net ionic equations.
- What is the difference between these types of equations?
- In what circumstance would the complete and net ionic equations for a reaction be identical?
Q4.1.3
Balance the following equations:
- \(\ce{PCl5}(s)+\ce{H2O}(l)\rightarrow \ce{POCl3}(l)+\ce{HCl}(aq)\)
- \(\ce{Cu}(s)+\ce{HNO3}(aq)\rightarrow \ce{Cu(NO3)2}(aq)+\ce{H2O}(l)+\ce{NO}(g)\)
- \(\ce{H2}(g)+\ce{I2}(s)\rightarrow \ce{HI}(s)\)
- \(\ce{Fe}(s)+\ce{O2}(g)\rightarrow \ce{Fe2O3}(s)\)
- \(\ce{Na}(s)+\ce{H2O}(l)\rightarrow \ce{NaOH}(aq)+\ce{H2}(g)\)
- \(\ce{(NH4)2Cr2O7}(s)\rightarrow \ce{Cr2O3}(s)+\ce{N2}(g)+\ce{H2O}(g)\)
- \(\ce{P4}(s)+\ce{Cl2}(g)\rightarrow \ce{PCl3}(l)\)
- \(\ce{PtCl4}(s)\rightarrow \ce{Pt}(s)+\ce{Cl2}(g)\)
S4.1.3
- \(\ce{PCl5}(s)+\ce{H2O}(l)\rightarrow \ce{POCl3}(l)+\ce{2HCl}(aq)\);
- \(\ce{3Cu}(s)+\ce{8HNO3}(aq)\rightarrow \ce{3Cu(NO3)2}(aq)+\ce{4H2O}(l)+\ce{2NO}(g)\);
- \(\ce{H2}(g)+\ce{I2}(s)\rightarrow \ce{2HI}(s)\) ;
- \(\ce{4Fe}(s)+\ce{3O2}(g)\rightarrow \ce{2Fe2O3}(s)\);
- \(\ce{2Na}(s)+\ce{2H2O}(l)\rightarrow \ce{2NaOH}(aq)+\ce{H2}(g)\);
- \(\ce{(NH4)2Cr52O7}(s)\rightarrow \ce{Cr2O3}(s)+\ce{N2}(g)+\ce{4H2O}(g)\);
- \(\ce{P4}(s)+\ce{6Cl2}(g)\rightarrow \ce{4PCl3}(l)\) ;
- \(\ce{PtCl4}(s)\rightarrow \ce{Pt}(s)+\ce{2Cl2}(g)\)
Q4.1.4
Balance the following equations:
- \(\ce{Ag}(s)+\ce{H2S}(g)+\ce{O2}(g)\rightarrow \ce{Ag2S}(s)+\ce{H2O}(l)\)
- \(\ce{P4}(s)+\ce{O2}(g)\rightarrow \ce{P4O10}(s)\)
- \(\ce{Pb}(s)+\ce{H2O}(l)+\ce{O2}(g)\rightarrow \ce{Pb(OH)2}(s)\)
- \(\ce{Fe}(s)+\ce{H2O}(l)\rightarrow \ce{Fe3O4}(s)+\ce{H2}(g)\)
- \(\ce{Sc2O3}(s)+\ce{SO3}(l)\rightarrow \ce{Sc2(SO4)3}(s)\)
- \(\ce{Ca3(PO4)2}(aq)+\ce{H3PO4}(aq)\rightarrow \ce{Ca(H2PO4)2}(aq)\)
- \(\ce{Al}(s)+\ce{H2SO4}(aq)\rightarrow \ce{Al2(SO4)3}(s)+\ce{H2}(g)\)
- \(\ce{TiCl4}(s)+\ce{H2O}(g)\rightarrow \ce{TiO2}(s)+\ce{HCl}(g)\)
S4.1.4
- \(\ce{4Ag}(s)+\ce{2H2S}(g)+\ce{O2}(g)\rightarrow \ce{2Ag2S}(s)+\ce{2H2O}(l)\)
- \(\ce{P4}(s)+\ce{5O2}(g)\rightarrow \ce{P4O10}(s)\)
- \(\ce{2Pb}(s)+\ce{2H2O}(l)+\ce{O2}(g)\rightarrow \ce{2Pb(OH)2}(s)\)
- \(\ce{3Fe}(s)+\ce{4H2O}(l)\rightarrow \ce{Fe3O4}(s)+\ce{4H2}(g)\)
- \(\ce{Sc2O3}(s)+\ce{3SO3}(l)\rightarrow \ce{Sc2(SO4)3}(s)\)
- \(\ce{Ca3(PO4)2}(aq)+\ce{4H3PO4}(aq)\rightarrow \ce{3Ca(H2PO4)2}(aq)\)
- \(\ce{2Al}(s)+\ce{3H2SO4}(aq)\rightarrow \ce{Al2(SO4)3}(s)+\ce{3H2}(g)\)
- \(\ce{TiCl4}(s)+\ce{2H2O}(g)\rightarrow \ce{TiO2}(s)+\ce{4HCl}(g)\)
Q4.1.5
Write a balanced molecular equation describing each of the following chemical reactions.
- Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas.
- Gaseous butane, C4H10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor.
- Aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride.
- Water vapor reacts with sodium metal to produce solid sodium hydroxide and hydrogen gas.
S4.1.5
- \(\ce{CaCO3}(s)\rightarrow \ce{CaO}(s)+\ce{CO2}(g)\);
- \(\ce{2C4H10}(g)+\ce{13O2}(g)\rightarrow \ce{8CO2}(g)+\ce{10H2O}(g)\);
- \(\ce{MgCl2}(aq)+\ce{2NaOH}(aq)\rightarrow \ce{Mg(OH)2}(s)+\ce{2NaCl}(aq)\);
- \(\ce{2H2O}(g)+\ce{2Na}(s)\rightarrow \ce{2NaOH}(s)+\ce{H2}(g)\)
Q4.1.6
Write a balanced equation describing each of the following chemical reactions.
- Solid potassium chlorate, KClO3, decomposes to form solid potassium chloride and diatomic oxygen gas.
- Solid aluminum metal reacts with solid diatomic iodine to form solid Al2I6.
- When solid sodium chloride is added to aqueous sulfuric acid, hydrogen chloride gas and aqueous sodium sulfate are produced.
- Aqueous solutions of phosphoric acid and potassium hydroxide react to produce aqueous potassium dihydrogen phosphate and liquid water.
Q4.1.7
Colorful fireworks often involve the decomposition of barium nitrate and potassium chlorate and the reaction of the metals magnesium, aluminum, and iron with oxygen.
- Write the formulas of barium nitrate and potassium chlorate.
- The decomposition of solid potassium chlorate leads to the formation of solid potassium chloride and diatomic oxygen gas. Write an equation for the reaction.
- The decomposition of solid barium nitrate leads to the formation of solid barium oxide, diatomic nitrogen gas, and diatomic oxygen gas. Write an equation for the reaction.
- Write separate equations for the reactions of the solid metals magnesium, aluminum, and iron with diatomic oxygen gas to yield the corresponding metal oxides. (Assume the iron oxide contains Fe3+ ions.)
Q4.1.7
- Ba(NO3)2, KClO3;
- \(\ce{2KClO3}(s)\rightarrow \ce{2KCl}(s)+\ce{3O2}(g)\);
- \(\ce{2Ba(NO3)2}(s)\rightarrow \ce{2BaO}(s)+\ce{2N2}(g)+\ce{5O2}(g)\);
- \(\ce{2Mg}(s)+\ce{O2}(g)\rightarrow \ce{2MgO}(s)\) ; \(\ce{4Al}(s)+\ce{3O2}(g)\rightarrow \ce{2Al2O3}(g)\); \(\ce{4Fe}(s)+\ce{3O2}(g)\rightarrow \ce{2Fe2O3}(s)\)
Q4.1.8
Fill in the blank with a single chemical formula for a covalent compound that will balance the equation:
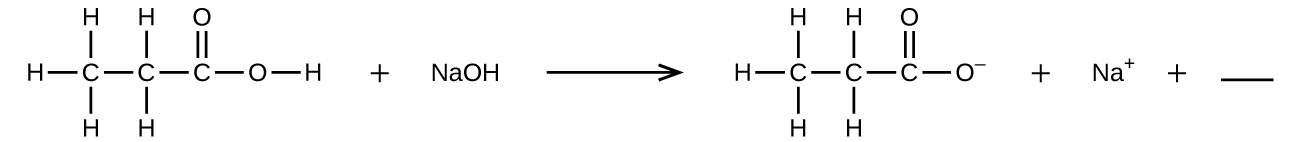
Q4.1.9
Aqueous hydrogen fluoride (hydrofluoric acid) is used to etch glass and to analyze minerals for their silicon content. Hydrogen fluoride will also react with sand (silicon dioxide).
- Write an equation for the reaction of solid silicon dioxide with hydrofluoric acid to yield gaseous silicon tetrafluoride and liquid water.
- The mineral fluorite (calcium fluoride) occurs extensively in Illinois. Solid calcium fluoride can also be prepared by the reaction of aqueous solutions of calcium chloride and sodium fluoride, yielding aqueous sodium chloride as the other product. Write complete and net ionic equations for this reaction.
S4.1.9
- \(\ce{4HF}(aq)+\ce{SiO2}(s)\rightarrow \ce{SiF4}(g)+\ce{2H2O}(l)\);
- complete ionic equation: \(\ce{2Na+}(aq)+\ce{2F-}(aq)+\ce{Ca^2+}(aq)+\ce{2Cl-}(aq)\rightarrow \ce{CaF2}(s)+\ce{2Na+}(aq)+\ce{2Cl-}(aq)\), net ionic equation: \(\ce{2F-}(aq)+\ce{Ca^2+}(aq)\rightarrow \ce{CaF2}(s)\)
Q4.1.10
A novel process for obtaining magnesium from sea water involves several reactions. Write a balanced chemical equation for each step of the process.
- The first step is the decomposition of solid calcium carbonate from seashells to form solid calcium oxide and gaseous carbon dioxide.
- The second step is the formation of solid calcium hydroxide as the only product from the reaction of the solid calcium oxide with liquid water.
- Solid calcium hydroxide is then added to the seawater, reacting with dissolved magnesium chloride to yield solid magnesium hydroxide and aqueous calcium chloride.
- The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water.
- Finally, the magnesium chloride is melted and electrolyzed to yield liquid magnesium metal and diatomic chlorine gas.
Q4.1.11
From the balanced molecular equations, write the complete ionic and net ionic equations for the following:
- \(\ce{K2C2O4}(aq)+\ce{Ba(OH)2}(aq)\rightarrow \ce{2KOH}(aq)+\ce{BaC2O2}(s)\)
- \(\ce{Pb(NO3)2}(aq)+\ce{H2SO4}(aq)\rightarrow \ce{PbSO4}(s)+\ce{2HNO3}(aq)\)
- \(\ce{CaCO3}(s)+\ce{H2SO4}(aq)\rightarrow \ce{CaSO4}(s)+\ce{CO2}(g)+\ce{H2O}(l)\)
S4.1.11
- \[\ce{2K+}(aq)+\ce{C2O4^2-}(aq)+\ce{Ba^2+}(aq)+\ce{2OH-}(aq)\rightarrow \ce{2K+}(aq)+\ce{2OH-}(aq)+\ce{BaC2O4}(s)\hspace{20px}\ce{(complete)}\] \[\ce{Ba^2+}(aq)+\ce{C2O4^2-}(aq)\rightarrow \ce{BaC2O4}(s)\hspace{20px}\ce{(net)}\]
- \[\ce{Pb^2+}(aq)+\ce{2NO3-}(aq)+\ce{2H+}(aq)+\ce{SO4^2-}(aq)\rightarrow \ce{PbSO4}(s)+\ce{2H+}(aq)+\ce{2NO3-}(aq)\hspace{20px}\ce{(complete)}\]\[\ce{Pb^2+}(aq)+\ce{SO4^2-}(aq)\rightarrow \ce{PbSO4}(s)\hspace{20px}\ce{(net)}\]
- \[\ce{CaCO3}(s)+\ce{2H+}(aq)+\ce{SO4^2-}(aq)\rightarrow \ce{CaSO4}(s)+\ce{CO2}(g)+\ce{H2O}(l)\hspace{20px}\ce{(complete)}\]\[\ce{CaCO3}(s)+\ce{2H+}(aq)+\ce{SO4^2-}(aq)\rightarrow \ce{CaSO4}(s)+\ce{CO2}(g)+\ce{H2O}(l)\hspace{20px}\ce{(net)}\]
4.2: Classifying Chemical Reactions
Q4.2.1
Use the following equations to answer the next five questions:
- \(\ce{H2O}(s)\rightarrow \ce{H2O}(l)\)
- \(\ce{Na+}(aq)+\ce{Cl-}(aq)\ce{Ag+}(aq)+\ce{NO3-}(aq) \rightarrow \ce{AgCl}(s)+\ce{Na+}(aq)+\ce{NO3-}(aq)\)
- \(\ce{CH3OH}(g)+\ce{O2}(g)\rightarrow \ce{CO2}(g)+\ce{H2O}(g)\)
- \(\ce{2H2O}(l)\rightarrow \ce{2H2}(g)+\ce{O2}(g)\)
- \(\ce{H+}(aq)+\ce{OH-}(aq)\rightarrow \ce{H2O}(l)\)
- Which equation describes a physical change?
- Which equation identifies the reactants and products of a combustion reaction?
- Which equation is not balanced?
- Which is a net ionic equation?
S4.2.1
a.) i. \(H_2O (solid) → H_2O(liquid)\)
b.) iii.
c.) iii. \(\ce{2CH3OH}(g)+\ce{3O2}(g)\rightarrow \ce{2CO2}(g)+\ce{4H2O}(g)\)
d.) v.
Q4.2.2
Indicate what type, or types, of reaction each of the following represents:
- \(\ce{Ca}(s)+\ce{Br2}(l)\rightarrow \ce{CaBr2}(s)\)
- \(\ce{Ca(OH)2}(aq)+\ce{2HBr}(aq)\rightarrow \ce{CaBr2}(aq)+\ce{2H2O}(l)\)
- \(\ce{C6H12}(l)+\ce{9O2}(g)\rightarrow \ce{6CO2}(g)+\ce{6H2O}(g)\)
S4.2.2
oxidation-reduction (addition); acid-base (neutralization); oxidation-reduction (combustion)
<
Q4.2.3
Indicate what type, or types, of reaction each of the following represents:
- \(\ce{H2O}(g)+\ce{C}(s)\rightarrow \ce{CO}(g)+\ce{H2}(g)\)
- \(\ce{2KClO3}(s)\rightarrow \ce{2KCl}(s)+\ce{3O2}(g)\)
- \(\ce{Al(OH)3}(aq)+\ce{3HCl}(aq)\rightarrow \ce{AlBr3}(aq)+\ce{3H2O}(l)\)
- \(\ce{Pb(NO3)2}(aq)+\ce{H2SO4}(aq)\rightarrow \ce{PbSO4}(s)+\ce{2HNO3}(aq)\)
Q4.2.4
Silver can be separated from gold because silver dissolves in nitric acid while gold does not. Is the dissolution of silver in nitric acid an acid-base reaction or an oxidation-reduction reaction? Explain your answer.
S4.2.4
It is an oxidation-reduction reaction because the oxidation state of the silver changes during the reaction.
Q4.2.5
Determine the oxidation states of the elements in the following compounds:
- NaI
- GdCl3
- LiNO3
- H2Se
- Mg2Si
- RbO2, rubidium superoxide
- HF
Q4.2.6
Determine the oxidation states of the elements in the compounds listed. None of the oxygen-containing compounds are peroxides or superoxides.
- H3PO4
- Al(OH)3
- SeO2
- KNO2
- In2S3
- P4O6
S4.2.6
H +1, P +5, O −2; Al +3, H +1, O −2; Se +4, O −2; K +1, N +3, O −2; In +3, S −2; P +3, O −2
Q4.2.7
Determine the oxidation states of the elements in the compounds listed. None of the oxygen-containing compounds are peroxides or superoxides.
- H2SO4
- Ca(OH)2
- BrOH
- ClNO2
- TiCl4
- NaH
S4.2.7
- H1+, O2-, S6+
- H1+, O2-, Ca+2
- H1+, O2-, Br1+
- O2-, Cl1-, N5+
- Cl1-, Ti4+
- H1+, Na1-
Q4.2.8
Classify the following as acid-base reactions or oxidation-reduction reactions:
- \(\ce{Na2S}(aq)+\ce{2HCl}(aq)\rightarrow \ce{2NaCl}(aq)+\ce{H2S}(g)\)
- \(\ce{2Na}(s)+\ce{2HCl}(aq)\rightarrow \ce{2NaCl}(aq)+\ce{H2}(g)\)
- \(\ce{Mg}(s)+\ce{Cl2}(g)\rightarrow \ce{MgCl2}(s)\)
- \(\ce{MgO}(s)+\ce{2HCl}(aq)\rightarrow \ce{MgCl2}(aq)+\ce{H2O}(l)\)
- \(\ce{K3P}(s)+\ce{2O2}(g)\rightarrow \ce{K3PO4}(s)\)
- \(\ce{3KOH}(aq)+\ce{H3PO4}(aq)\rightarrow \ce{K3PO4}(aq)+\ce{3H2O}(l)\)
S4.2.9
acid-base; oxidation-reduction: Na is oxidized, H+ is reduced; oxidation-reduction: Mg is oxidized, Cl2 is reduced; acid-base; oxidation-reduction: P3− is oxidized, O2 is reduced; acid-base
Q4.2.10
Identify the atoms that are oxidized and reduced, the change in oxidation state for each, and the oxidizing and reducing agents in each of the following equations:
- \(\ce{Mg}(s)+\ce{NiCl2}(aq)\rightarrow \ce{MgCl2}(aq)+\ce{Ni}(s)\)
- \(\ce{PCl3}(l)+\ce{Cl2}(g)\rightarrow \ce{PCl5}(s)\)
- \(\ce{C2H4}(g)+\ce{3O2}(g)\rightarrow \ce{2CO2}(g)+\ce{2H2O}(g)\)
- \(\ce{Zn}(s)+\ce{H2SO4}(aq)\rightarrow \ce{ZnSO4}(aq)+\ce{H2}(g)\)
- \(\ce{2K2S2O3}(s)+\ce{I2}(s)\rightarrow \ce{K2S4O6}(s)+\ce{2KI}(s)\)
- \(\ce{3Cu}(s)+\ce{8HNO3}(aq)\rightarrow\ce{3Cu(NO3)2}(aq)+\ce{2NO}(g)+\ce{4H2O}(l)\)
Q4.2.11
Complete and balance the following acid-base equations:
- HCl gas reacts with solid Ca(OH)2(s).
- A solution of Sr(OH)2 is added to a solution of HNO3.
S4.2.11
- \(\ce{2HCl}(g)+\ce{Ca(OH)2}(s)\rightarrow \ce{CaCl2}(s)+\ce{2H2O}(l)\);
- \(\ce{Sr(OH)2}(aq)+\ce{2HNO3}(aq)\rightarrow \ce{Sr(NO3)2}(aq)+\ce{2H2O}(l)\)
Q4.2.12
Complete and balance the following acid-base equations:
- A solution of HClO4 is added to a solution of LiOH.
- Aqueous H2SO4 reacts with NaOH.
- Ba(OH)2 reacts with HF gas.
Q4.2.13
Complete and balance the following oxidation-reduction reactions, which give the highest possible oxidation state for the oxidized atoms.
- \(\ce{Al}(s)+\ce{F2}(g)\rightarrow\)
- \(\ce{Al}(s)+\ce{CuBr2}(aq)\rightarrow\) (single displacement)
- \(\ce{P4}(s)+\ce{O2}(g)\rightarrow \)
- \(\ce{Ca}(s)+\ce{H2O}(l)\rightarrow \) (products are a strong base and a diatomic gas)
S4.2.13
- \(\ce{2Al}(s)+\ce{3F2}(g)\rightarrow \ce{2AlF3}(s)\);
- \(\ce{2Al}(s)+\ce{3CuBr2}(aq)\rightarrow \ce{3Cu}(s)+\ce{2AlBr3}(aq)\);
- \(\ce{P4}(s)+\ce{5O2}(g)\rightarrow \ce{P4O10}(s)\); \(\ce{Ca}(s)+\ce{2H2O}(l)\rightarrow \ce{Ca(OH)2}(aq)+\ce{H2}(g)\)
Q4.2.14
Complete and balance the following oxidation-reduction reactions, which give the highest possible oxidation state for the oxidized atoms.
- \(\ce{K}(s)+\ce{H2O}(l)\rightarrow \)
- \(\ce{Ba}(s)+\ce{HBr}(aq)\rightarrow \)
- \(\ce{Sn}(s)+\ce{I2}(s)\rightarrow \)
Q4.2.15
Complete and balance the equations for the following acid-base neutralization reactions. If water is used as a solvent, write the reactants and products as aqueous ions. In some cases, there may be more than one correct answer, depending on the amounts of reactants used.
- \(\ce{Mg(OH)2}(s)+\ce{HClO4}(aq)\rightarrow \)
- \(\ce{SO3}(g)+\ce{H2O}(l)\rightarrow \) (assume an excess of water and that the product dissolves)
- \(\ce{SrO}(s)+\ce{H2SO4}(l)\rightarrow \)
S4.2.15
- \(\ce{Mg(OH)2}(s)+\ce{2HClO4}(aq)\rightarrow \ce{Mg^2+}(aq)+\ce{2ClO4-}(aq)+\ce{2H2O}(l)\) ;
- \(\ce{SO3}(g)+\ce{2H2O}(l)\rightarrow \ce{H3O+}(aq)+\ce{HSO4-}(aq)\), (a solution of H2SO4);
- \(\ce{SrO}(s)+\ce{H2SO4}(l)\rightarrow \ce{SrSO4}(s)+\ce{H2O}\)
Q4.2.16
When heated to 700–800 °C, diamonds, which are pure carbon, are oxidized by atmospheric oxygen. (They burn!) Write the balanced equation for this reaction.
Q4.2.17
The military has experimented with lasers that produce very intense light when fluorine combines explosively with hydrogen. What is the balanced equation for this reaction?
S4.2.17
\(\ce{H2}(g)+\ce{F2}(g)\rightarrow \ce{2HF}(g)\)
Q4.2.18
Write the molecular, total ionic, and net ionic equations for the following reactions:
- \(\ce{Ca(OH)2}(aq)+\ce{HC2H3O2}(aq)\rightarrow \)
- \(\ce{H3PO4}(aq)+\ce{CaCl2}(aq)\rightarrow \)
Q4.2.19
Great Lakes Chemical Company produces bromine, Br2, from bromide salts such as NaBr, in Arkansas brine by treating the brine with chlorine gas. Write a balanced equation for the reaction of NaBr with Cl2.
S4.2.19
\(\ce{2NaBr}(aq)+\ce{Cl2}(g)\rightarrow \ce{2NaCl}(aq)+\ce{Br2}(l)\)
Q4.2.20
In a common experiment in the general chemistry laboratory, magnesium metal is heated in air to produce MgO. MgO is a white solid, but in these experiments it often looks gray, due to small amounts of Mg3N2, a compound formed as some of the magnesium reacts with nitrogen. Write a balanced equation for each reaction.
Q4.2.21
Lithium hydroxide may be used to absorb carbon dioxide in enclosed environments, such as manned spacecraft and submarines. Write an equation for the reaction that involves 2 mol of LiOH per 1 mol of CO2. (Hint: Water is one of the products.)
S4.2.21
\(\ce{2LiOH}(aq)+\ce{CO2}(g)\rightarrow \ce{Li2CO3}(aq)+\ce{H2O}(l)\)
Q4.2.22
Calcium propionate is sometimes added to bread to retard spoilage. This compound can be prepared by the reaction of calcium carbonate, CaCO3, with propionic acid, C2H5CO2H, which has properties similar to those of acetic acid. Write the balanced equation for the formation of calcium propionate.
Q4.2.23
Complete and balance the equations of the following reactions, each of which could be used to remove hydrogen sulfide from natural gas:
- \(\ce{Ca(OH)2}(s)+\ce{H2S}(g) \rightarrow\)
- \(\ce{Na2CO3}(aq)+\ce{H2S}(g)\rightarrow \)
S4.2.23
- \(\ce{Ca(OH)2}(s)+\ce{H2S}(g)\rightarrow \ce{CaS}(s)+\ce{2H2O}(l)\);
- \(\ce{Na2CO3}(aq)+\ce{H2S}(g)\rightarrow \ce{Na2S}(aq)+\ce{CO2}(g)+\ce{H2O}(l)\)
Q4.2.24
Copper(II) sulfide is oxidized by molecular oxygen to produce gaseous sulfur trioxide and solid copper(II) oxide. The gaseous product then reacts with liquid water to produce liquid hydrogen sulfate as the only product. Write the two equations which represent these reactions.
Q4.2.25
Write balanced chemical equations for the reactions used to prepare each of the following compounds from the given starting material(s). In some cases, additional reactants may be required.
- solid ammonium nitrate from gaseous molecular nitrogen via a two-step process (first reduce the nitrogen to ammonia, then neutralize the ammonia with an appropriate acid)
- gaseous hydrogen bromide from liquid molecular bromine via a one-step redox reaction
- gaseous H2S from solid Zn and S via a two-step process (first a redox reaction between the starting materials, then reaction of the product with a strong acid)
S4.2.25
- step 1: \(\ce{N2}(g)+\ce{3H2}(g)\rightarrow \ce{2NH3}(g)\), step 2: \(\ce{NH3}(g)+\ce{HNO3}(aq)\rightarrow \ce{NH4NO3}(aq)\rightarrow \ce{NH4NO3}(s)\ce{(after\: drying)}\);
- \(\ce{H2}(g)+\ce{Br2}(l)\rightarrow \ce{2HBr}(g)\);
- \(\ce{Zn}(s)+\ce{S}(s)\rightarrow \ce{ZnS}(s)\) and \(\ce{ZnS}(s)+\ce{2HCl}(aq)\rightarrow \ce{ZnCl2}(aq)+\ce{H2S}(g)\)
Q4.2.26
Calcium cyclamate Ca(C6H11NHSO3)2 is an artificial sweetener used in many countries around the world but is banned in the United States. It can be purified industrially by converting it to the barium salt through reaction of the acid C6H11NHSO3H with barium carbonate, treatment with sulfuric acid (barium sulfate is very insoluble), and then neutralization with calcium hydroxide. Write the balanced equations for these reactions.
Q4.2.27
Complete and balance each of the following half-reactions (steps 2–5 in half-reaction method):
- \(\ce{Sn^4+}(aq)\rightarrow \ce{Sn^2+}(aq)\)
- \(\ce{[Ag(NH3)2]+}(aq)\rightarrow \ce{Ag}(s)+\ce{NH3}(aq)\)
- \(\ce{Hg2Cl2}(s)\rightarrow \ce{Hg}(l)+\ce{Cl-}(aq)\)
- \(\ce{H2O}(l)\rightarrow \ce{O2}(g)\ce{\:(in\: acidic\: solution)}\)
- \(\ce{IO3-}(aq)\rightarrow \ce{I2}(s)\)
- \(\ce{SO3^2-}(aq)\rightarrow \ce{SO4^2-}(aq)\ce{\:(in\: acidic\: solution)}\)
- \(\ce{MnO4-}(aq)\rightarrow \ce{Mn^2+}(aq)\ce{\:(in\: acidic\: solution)}\)
- \(\ce{Cl-}(aq)\rightarrow \ce{ClO3-}(aq)\ce{\:(in\: basic\: solution)}\)
S4.2.27
- \(\ce{Sn^4+}(aq)+\ce{2e-}\rightarrow \ce{Sn^2+}(aq)\),
- \(\ce{[Ag(NH3)2]+}(aq)+ \ce{e-} \rightarrow \ce{Ag}(s)+\ce{2NH3}(aq)\);
- \(\ce{Hg2Cl2}(s)+ \ce{2e-} \rightarrow \ce{2Hg}(l)+\ce{2Cl-}(aq)\) ;
- \(\ce{2H2O}(l)\rightarrow \ce{O2}(g)+\ce{4H+}(aq)+\ce{4e-}\);
- \(\ce{6H2O}(l)+\ce{2IO3-}(aq)+\ce{10e-}\rightarrow \ce{I2}(s)+\ce{12OH-}(aq)\);
- \(\ce{H2O}(l)+\ce{SO3^2-}(aq)\rightarrow \ce{SO4^2-}(aq)+\ce{2H+}(aq)+\ce{2e-}\);
- (g) \(\ce{8H+}(aq)+\ce{MnO4-}(aq)+\ce{5e-}\rightarrow \ce{Mn^2+}(aq)+\ce{4H2O}(l)\);
- (h) \(\ce{Cl-}(aq)+\ce{6OH-}(aq)\rightarrow \ce{ClO3-}(aq)+\ce{3H2O}(l)+\ce{6e-}\)
Q4.2.28
Complete and balance each of the following half-reactions (steps 2–5 in half-reaction method):
- \(\ce{Cr^2+}(aq)\rightarrow \ce{Cr^3+}(aq)\)
- \(\ce{Hg}(l)+\ce{Br-}(aq)\rightarrow \ce{HgBr4^2-}(aq)\)
- \(\ce{ZnS}(s)\rightarrow \ce{Zn}(s)+\ce{S^2-}(aq)\)
- \(\ce{H2}(g)\rightarrow \ce{H2O}(l)\ce{\:(in\: basic\: solution)}\)
- \(\ce{H2}(g)\rightarrow \ce{H3O+}(aq)\ce{\:(in\: acidic\: solution)}\)
- \(\ce{NO3-}(aq)\rightarrow \ce{HNO2}(aq)\ce{\:(in\: acidic\: solution)}\)
- \(\ce{MnO2}(s)\rightarrow \ce{MnO4-}(aq)\ce{\:(in\: basic\: solution)}\)
- \(\ce{Cl-}(aq)\rightarrow \ce{ClO3-}(aq)\ce{\:(in\: acidic\: solution)}\)
Q4.2.29
Balance each of the following equations according to the half-reaction method:
- \(\ce{Sn^2+}(aq)+\ce{Cu^2+}(aq)\rightarrow \ce{Sn^4+}(aq)+\ce{Cu+}(aq)\)
- \(\ce{H2S}(g)+\ce{Hg2^2+}(aq)\rightarrow \ce{Hg}(l)+\ce{S}(s)\ce{\:(in\: acid)}\)
- \(\ce{CN-}(aq)+\ce{ClO2}(aq)\rightarrow \ce{CNO-}(aq)+\ce{Cl-}(aq)\ce{\:(in\: acid)}\)
- \(\ce{Fe^2+}(aq)+\ce{Ce^4+}(aq)\rightarrow \ce{Fe^3+}(aq)+\ce{Ce^3+}(aq)\)
- \(\ce{HBrO}(aq)\rightarrow \ce{Br-}(aq)+\ce{O2}(g)\ce{\:(in\: acid)}\)
S4.2.29
- \(\ce{Sn^2+}(aq)+\ce{2Cu^2+}(aq)\rightarrow \ce{Sn^4+}(aq)+\ce{2Cu+}(aq)\);
- \(\ce{H2S}(g)+\ce{Hg2^2+}(aq)+\ce{2H2O}(l)\rightarrow \ce{2Hg}(l)+\ce{S}(s)+\ce{2H3O+}(aq)\);
- \(\ce{5CN-}(aq)+\ce{2ClO2}(aq)+\ce{3H2O}(l)\rightarrow \ce{5CNO-}(aq)+\ce{2Cl-}(aq)+\ce{2H3O+}(aq)\);
- \(\ce{Fe^2+}(aq)+\ce{Ce^4+}(aq)\rightarrow \ce{Fe^3+}(aq)+\ce{Ce^3+}(aq)\);
- \(\ce{2HBrO}(aq)+\ce{2H2O}(l)\rightarrow \ce{2H3O+}(aq)+\ce{2Br-}(aq)+\ce{O2}(g)\)
Q4.2.30
Balance each of the following equations according to the half-reaction method:
- \(\ce{Zn}(s)+\ce{NO3-}(aq)\rightarrow \ce{Zn^2+}(aq)+\ce{N2}(g)\ce{\:(in\: acid)}\)
- \(\ce{Zn}(s)+\ce{NO3-}(aq)\rightarrow \ce{Zn^2+}(aq)+\ce{NH3}(aq)\ce{\:(in\: base)}\)
- \(\ce{CuS}(s)+\ce{NO3-}(aq)\rightarrow \ce{Cu^2+}(aq)+\ce{S}(s)+\ce{NO}(g)\ce{\:(in\: acid)}\)
- \(\ce{NH3}(aq)+\ce{O2}(g)\rightarrow \ce{NO2}(g)\ce{\:(gas\: phase)}\)
- \(\ce{Cl2}(g)+\ce{OH-}(aq)\rightarrow \ce{Cl-}(aq)+\ce{ClO3-}(aq)\ce{\:(in\: base)}\)
- \(\ce{H2O2}(aq)+\ce{MnO4-}(aq)\rightarrow \ce{Mn^2+}(aq)+\ce{O2}(g)\ce{\:(in\: acid)}\)
- \(\ce{NO2}(g)\rightarrow \ce{NO3-}(aq)+\ce{NO2-}(aq)\ce{\:(in\: base)}\)
- \(\ce{Fe^3+}(aq)+\ce{I-}(aq)\rightarrow \ce{Fe^2+}(aq)+\ce{I2}(aq)\)
Q4.2.31
Balance each of the following equations according to the half-reaction method:
- \(\ce{MnO4-}(aq)+\ce{NO2-}(aq)\rightarrow \ce{MnO2}(s)+\ce{NO3-}(aq)\ce{\:(in\: base)}\)
- \(\ce{MnO4^2-}(aq)\rightarrow \ce{MnO4-}(aq)+\ce{MnO2}(s)\ce{\:(in\: base)}\)
- \(\ce{Br2}(l)+\ce{SO2}(g)\rightarrow \ce{Br-}(aq)+\ce{SO4^2-}(aq)\ce{\:(in\: acid)}\)
S4.2.31
- \(\ce{2MnO4-}(aq)+\ce{3NO2-}(aq)+\ce{H2O}(l)\rightarrow \ce{2MnO2}(s)+\ce{3NO3-}(aq)+\ce{2OH-}(aq)\);
- \(\ce{3MnO4^2-}(aq)+\ce{2H2O}(l)\rightarrow \ce{2MnO4-}(aq)+\ce{4OH-}(aq)+\ce{MnO2}(s)\ce{\:(in\: base)}\);
- \(\ce{Br2}(l)+\ce{SO2}(g)+\ce{2H2O}(l)\rightarrow \ce{4H+}(aq)+\ce{2Br-}(aq)+\ce{SO4^2-}(aq)\)
4.3: Reaction Stoichiometry
Q4.3.1
Write the balanced equation, then outline the steps necessary to determine the information requested in each of the following:
- The number of moles and the mass of chlorine, Cl2, required to react with 10.0 g of sodium metal, Na, to produce sodium chloride, NaCl.
- The number of moles and the mass of oxygen formed by the decomposition of 1.252 g of mercury(II) oxide.
- The number of moles and the mass of sodium nitrate, NaNO3, required to produce 128 g of oxygen. (NaNO2 is the other product.)
- The number of moles and the mass of carbon dioxide formed by the combustion of 20.0 kg of carbon in an excess of oxygen.
- The number of moles and the mass of copper(II) carbonate needed to produce 1.500 kg of copper(II) oxide. (CO2 is the other product.)
-

Q4.3.2
Determine the number of moles and the mass requested for each reaction in Exercise.
S4.3.2
0.435 mol Na, 0.217 mol Cl2, 15.4 g Cl2; 0.005780 mol HgO, 2.890 × 10−3 mol O2, 9.248 × 10−2 g O2; 8.00 mol NaNO3, 6.8 × 102 g NaNO3; 1665 mol CO2, 73.3 kg CO2; 18.86 mol CuO, 2.330 kg CuCO3; 0.4580 mol C2H4Br2, 86.05 g C2H4Br2
Q4.3.3
Write the balanced equation, then outline the steps necessary to determine the information requested in each of the following:
- The number of moles and the mass of Mg required to react with 5.00 g of HCl and produce MgCl2 and H2.
- The number of moles and the mass of oxygen formed by the decomposition of 1.252 g of silver(I) oxide.
- The number of moles and the mass of magnesium carbonate, MgCO3, required to produce 283 g of carbon dioxide. (MgO is the other product.)
- The number of moles and the mass of water formed by the combustion of 20.0 kg of acetylene, C2H2, in an excess of oxygen.
- The number of moles and the mass of barium peroxide, BaO2, needed to produce 2.500 kg of barium oxide, BaO (O2 is the other product.)

Q4.3.4
Determine the number of moles and the mass requested for each reaction in Exercise.
S4.3.4
0.0686 mol Mg, 1.67 g Mg; 2.701 × 10−3 mol O2, 0.08644 g O2; 6.43 mol MgCO3, 542 g MgCO3 713 mol H2O, 12.8 kg H2O; 16.31 mol BaO2, 2762 g BaO2; 0.207 mol C2H4, 5.81 g C2H4
Q4.3.5
H2 is produced by the reaction of 118.5 mL of a 0.8775-M solution of H3PO4 according to the following equation: \(\ce{2Cr + 2H3PO4 \rightarrow 3H2 + 2CrPO4}\).
- Outline the steps necessary to determine the number of moles and mass of H2.
- Perform the calculations outlined.
S4.3.5
a.)
- Convert mL to L
- Multiply L by the molarity to determine moles of H3PO4
- Convert moles of H3PO4 to moles of H2
- Multiply moles of H2 by the molar mass of H2 to get the answer in grams
b.)
1. \(118.5\: mL\times \dfrac{1\: L}{1000\: mL} = 0.1185\: L\)
2. \(0.1185\: L \times \dfrac{0.8775\: moles\: \ce{H3PO4}}{1\: L} = 0.1040\: moles\: \ce{H3PO4}\)
3. \(0.1040\: moles\: \ce{H3PO4} \times \dfrac{3\: moles\:\ce{H_2}}{2\: moles\: \ce{H3PO4}} = 0.1560\: moles\: \ce{H2}\)
4. \(0.1560\: moles\: \ce{H2} \times \dfrac{2.02 g}{1\: mole} = 0.3151g\: \ce{H2}\)
Q4.3.6
Gallium chloride is formed by the reaction of 2.6 L of a 1.44 M solution of HCl according to the following equation: \(\ce{2Ga + 6HCl \rightarrow 2GaCl3 + 3H2}\).
- Outline the steps necessary to determine the number of moles and mass of gallium chloride.
- Perform the calculations outlined.
S4.3.6
\(\mathrm{volume\: HCl\: solution \rightarrow mol\: HCl \rightarrow mol\: GaCl_3}\); 1.25 mol GaCl3, 2.2 × 102 g GaCl3
Q4.3.7
I2 is produced by the reaction of 0.4235 mol of CuCl2 according to the following equation: \(\ce{2CuCl2 + 4KI \rightarrow 2CuI + 4KCl + I2}\).
- How many molecules of I2 are produced?
- What mass of I2 is produced?
Q4.3.8
Silver is often extracted from ores as K[Ag(CN)2] and then recovered by the reaction
\(\ce{2K[Ag(CN)2]}(aq)+\ce{Zn}(s)\rightarrow \ce{2Ag}(s)+\ce{Zn(CN)2}(aq)+\ce{2KCN}(aq)\)
- How many molecules of Zn(CN)2 are produced by the reaction of 35.27 g of K[Ag(CN)2]?
- What mass of Zn(CN)2 is produced?
S4.3.8
5.337 × 1022 molecules; 10.41 g Zn(CN)2
Q4.3.9
What mass of silver oxide, Ag2O, is required to produce 25.0 g of silver sulfadiazine, AgC10H9N4SO2, from the reaction of silver oxide and sulfadiazine?
\(\ce{2C10H10N4SO2 + Ag2O \rightarrow 2AgC10H9N4SO2 + H2O}\)
Q4.3.10
Carborundum is silicon carbide, SiC, a very hard material used as an abrasive on sandpaper and in other applications. It is prepared by the reaction of pure sand, SiO2, with carbon at high temperature. Carbon monoxide, CO, is the other product of this reaction. Write the balanced equation for the reaction, and calculate how much SiO2 is required to produce 3.00 kg of SiC.
S4.3.10
\(\ce{SiO2 + 3C \rightarrow SiC + 2CO}\), 4.50 kg SiO2
Q4.3.11
Automotive air bags inflate when a sample of sodium azide, NaN3, is very rapidly decomposed.
\(\ce{2NaN3}(s) \rightarrow \ce{2Na}(s) + \ce{3N2}(g)\)
What mass of sodium azide is required to produce 2.6 ft3 (73.6 L) of nitrogen gas with a density of 1.25 g/L?
S4.3.11
142g NaN3
Q4.3.12
Urea, CO(NH2)2, is manufactured on a large scale for use in producing urea-formaldehyde plastics and as a fertilizer. What is the maximum mass of urea that can be manufactured from the CO2 produced by combustion of 1.00×103 kg of carbon followed by the reaction?
\[\ce{CO2}(g)+\ce{2NH3}(g)\rightarrow \ce{CO(NH2)2}(s)+\ce{H2O}(l)\]
S4.3.12
5.00 × 103 kg
Q4.3.13
In an accident, a solution containing 2.5 kg of nitric acid was spilled. Two kilograms of Na2CO3 was quickly spread on the area and CO2 was released by the reaction. Was sufficient Na2CO3 used to neutralize all of the acid?
Q4.3.14
A compact car gets 37.5 miles per gallon on the highway. If gasoline contains 84.2% carbon by mass and has a density of 0.8205 g/mL, determine the mass of carbon dioxide produced during a 500-mile trip (3.785 liters per gallon).
S4.3.14
1.28 × 105 g CO2
Q4.3.15
What volume of a 0.750 M solution of hydrochloric acid, a solution of HCl, can be prepared from the HCl produced by the reaction of 25.0 g of NaCl with an excess of sulfuric acid?
\[\ce{NaCl}(s)+\ce{H2SO4}(l)\rightarrow \ce{HCl}(g)+\ce{NaHSO4}(s)\]
Q4.3.16
What volume of a 0.2089 M KI solution contains enough KI to react exactly with the Cu(NO3)2 in 43.88 mL of a 0.3842 M solution of Cu(NO3)2?
\[\ce{2Cu(NO3)2 + 4KI \rightarrow 2CuI + I2 + 4KNO3}\]
S4.3.16
161.40 mL KI solution
Q4.3.17
A mordant is a substance that combines with a dye to produce a stable fixed color in a dyed fabric. Calcium acetate is used as a mordant. It is prepared by the reaction of acetic acid with calcium hydroxide.
\[\ce{2CH3CO2H + Ca(OH)2 \rightarrow Ca(CH3CO2)2 + 2H2O}\]
What mass of Ca(OH)2 is required to react with the acetic acid in 25.0 mL of a solution having a density of 1.065 g/mL and containing 58.0% acetic acid by mass?
Q4.3.18
The toxic pigment called white lead, Pb3(OH)2(CO3)2, has been replaced in white paints by rutile, TiO2. How much rutile (g) can be prepared from 379 g of an ore that contains 88.3% ilmenite (FeTiO3) by mass?
\[\ce{2FeTiO3 + 4HCl + Cl2 \rightarrow 2FeCl3 + 2TiO2 + 2H2O}\]
S4.3.18
176 g TiO2
4.4: Reaction Yields
Q4.4.1
The following quantities are placed in a container: 1.5 × 1024 atoms of hydrogen, 1.0 mol of sulfur, and 88.0 g of diatomic oxygen.
- What is the total mass in grams for the collection of all three elements?
- What is the total number of moles of atoms for the three elements?
- If the mixture of the three elements formed a compound with molecules that contain two hydrogen atoms, one sulfur atom, and four oxygen atoms, which substance is consumed first?
- How many atoms of each remaining element would remain unreacted in the change described in ?
Q4.4.2
What is the limiting reactant in a reaction that produces sodium chloride from 8 g of sodium and 8 g of diatomic chlorine?
S4.4.2
The limiting reactant is Cl2.
Q4.4.3
Which of the postulates of Dalton's atomic theory explains why we can calculate a theoretical yield for a chemical reaction?
Q4.4.4
A student isolated 25 g of a compound following a procedure that would theoretically yield 81 g. What was his percent yield?
S4.4.4
\(\mathrm{Percent\: yield = 31\%}\)
Q4.4.5
A sample of 0.53 g of carbon dioxide was obtained by heating 1.31 g of calcium carbonate. What is the percent yield for this reaction?
\[\ce{CaCO3}(s)\rightarrow \ce{CaO}(s)+\ce{CO2}(s)\]
Q4.4.6
Freon-12, CCl2F2, is prepared from CCl4 by reaction with HF. The other product of this reaction is HCl. Outline the steps needed to determine the percent yield of a reaction that produces 12.5 g of CCl2F2 from 32.9 g of CCl4. Freon-12 has been banned and is no longer used as a refrigerant because it catalyzes the decomposition of ozone and has a very long lifetime in the atmosphere. Determine the percent yield.
S4.4.6
\(\ce{g\: CCl4\rightarrow mol\: CCl4\rightarrow mol\: CCl2F2 \rightarrow g\: CCl2F2}, \mathrm{\:percent\: yield=48.3\%}\)
Q4.4.7
Citric acid, C6H8O7, a component of jams, jellies, and fruity soft drinks, is prepared industrially via fermentation of sucrose by the mold Aspergillus niger. The equation representing this reaction is
\[\ce{C12H22O11 + H2O + 3O2 \rightarrow 2C6H8O7 + 4H2O}\]
What mass of citric acid is produced from exactly 1 metric ton (1.000 × 103 kg) of sucrose if the yield is 92.30%?
Q4.4.8
Toluene, C6H5CH3, is oxidized by air under carefully controlled conditions to benzoic acid, C6H5CO2H, which is used to prepare the food preservative sodium benzoate, C6H5CO2Na. What is the percent yield of a reaction that converts 1.000 kg of toluene to 1.21 kg of benzoic acid?
\[\ce{2C6H5CH3 + 3O2 \rightarrow 2C6H5CO2H + 2H2O}\]
S4.4.8
\(\mathrm{percent\: yield=91.3\%}\)
Q4.4.9
In a laboratory experiment, the reaction of 3.0 mol of H2 with 2.0 mol of I2 produced 1.0 mol of HI. Determine the theoretical yield in grams and the percent yield for this reaction.
Q4.4.10
Outline the steps needed to solve the following problem, then do the calculations. Ether, (C2H5)2O, which was originally used as an anesthetic but has been replaced by safer and more effective medications, is prepared by the reaction of ethanol with sulfuric acid.
2C2H5OH + H2SO4 ⟶ (C2H5)2 + H2SO4·H2O
Q4.4.11
What is the percent yield of ether if 1.17 L (d = 0.7134 g/mL) is isolated from the reaction of 1.500 L of C2H5OH (d = 0.7894 g/mL)?
S4.4.11
Convert mass of ethanol to moles of ethanol; relate the moles of ethanol to the moles of ether produced using the stoichiometry of the balanced equation. Convert moles of ether to grams; divide the actual grams of ether (determined through the density) by the theoretical mass to determine the percent yield; 87.6%
Q4.4.12
Outline the steps needed to determine the limiting reactant when 30.0 g of propane, C3H8, is burned with 75.0 g of oxygen.
\[\mathrm{percent\: yield=\dfrac{0.8347\:\cancel{g}}{0.9525\:\cancel{g}}\times 100\%=87.6\%}\]
Determine the limiting reactant.
Q4.4.13
Outline the steps needed to determine the limiting reactant when 0.50 g of Cr and 0.75 g of H3PO4 react according to the following chemical equation?
\[\ce{2Cr + 2H3PO4 \rightarrow 2CrPO4 + 3H2}\]
Determine the limiting reactant.
S4.4.13
The conversion needed is \(\ce{mol\: Cr \rightarrow mol\: H2PO4}\). Then compare the amount of Cr to the amount of acid present. Cr is the limiting reactant.
Q4.4.14
What is the limiting reactant when 1.50 g of lithium and 1.50 g of nitrogen combine to form lithium nitride, a component of advanced batteries, according to the following unbalanced equation?
\[\ce{Li + N2 \rightarrow Li3N}\]
S4.4.14
\[\ce{6Li} + \ce{N2} \rightarrow \: \ce{2Li3N}\]
\[1.50g\: \ce{Li} \times \dfrac{1\: mole\: \ce{Li}}{6.94g\: \ce{Li}} \times\dfrac{2\: mole\: \ce{Li3N}}{6\:mole\: \ce{Li}} = 0.0720\: moles\: \ce{Li3N}\]
\[1.50g\: \ce{N2} \times \dfrac{1\: mole\: \ce{N2}}{28.02g\: \ce{N2}} \times\dfrac{2\: mole\: \ce{Li3N}}{1\:mole\: \ce{N2}} = 0.107\: moles\: \ce{Li3N}\]
\(\ce{Li}\) is the limiting reactant
Q4.4.15
Uranium can be isolated from its ores by dissolving it as UO2(NO3)2, then separating it as solid UO2(C2O4)·3H2O. Addition of 0.4031 g of sodium oxalate, Na2C2O4, to a solution containing 1.481 g of uranyl nitrate, UO2(NO2)2, yields 1.073 g of solid UO2(C2O4)·3H2O.
\[\ce{Na2C2O4 + UO2(NO3)2 + 3H2O ⟶ UO2(C2O4)·3H2O + 2NaNO3}\]
Determine the limiting reactant and the percent yield of this reaction.
S4.4.15
Na2C2O4 is the limiting reactant. percent yield = 86.6%
Q4.4.16
How many molecules of C2H4Cl2 can be prepared from 15 C2H4 molecules and 8 Cl2 molecules?
Q4.4.17
How many molecules of the sweetener saccharin can be prepared from 30 C atoms, 25 H atoms, 12 O atoms, 8 S atoms, and 14 N atoms?
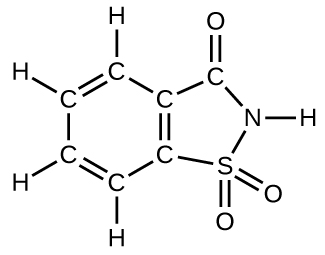
S4.4.17
Only four molecules can be made.
Q4.4.18
The phosphorus pentoxide used to produce phosphoric acid for cola soft drinks is prepared by burning phosphorus in oxygen.
- What is the limiting reactant when 0.200 mol of P4 and 0.200 mol of O2 react according to \[\ce{P4 + 5O2 \rightarrow P4O10}\]
- Calculate the percent yield if 10.0 g of P4O10 is isolated from the reaction.
Q4.4.19
Would you agree to buy 1 trillion (1,000,000,000,000) gold atoms for $5? Explain why or why not. Find the current price of gold at http://money.cnn.com/data/commodities/ \(\mathrm{(1\: troy\: ounce=31.1\: g)}\)
S4.4.19
This amount cannot be weighted by ordinary balances and is worthless.
4.5: Quantitative Chemical Analysis
Q4.5.1
What volume of 0.0105-M HBr solution is be required to titrate 125 mL of a 0.0100-M Ca(OH)2 solution?
\[\ce{Ca(OH)2}(aq)+\ce{2HBr}(aq) \rightarrow \ce{CaBr2}(aq)+\ce{2H2O}(l)\]
Q4.5.2
Titration of a 20.0-mL sample of acid rain required 1.7 mL of 0.0811 M NaOH to reach the end point. If we assume that the acidity of the rain is due to the presence of sulfuric acid, what was the concentration of sulfuric acid in this sample of rain?
S4.5.2
3.4 × 10−3 M H2SO4
Q4.5.3
What is the concentration of NaCl in a solution if titration of 15.00 mL of the solution with 0.2503 M AgNO3 requires 20.22 mL of the AgNO3 solution to reach the end point?
\[\ce{AgNO3}(aq)+\ce{NaCl}(aq)\rightarrow \ce{AgCl}(s)+\ce{NaNO3}(aq)\]
Q4.5.4
In a common medical laboratory determination of the concentration of free chloride ion in blood serum, a serum sample is titrated with a Hg(NO3)2 solution.
\[\ce{2Cl-}(aq)+\ce{Hg(NO3)2}(aq)\rightarrow \ce{2NO3-}(aq)+\ce{HgCl2}(s)\]
What is the Cl− concentration in a 0.25-mL sample of normal serum that requires 1.46 mL of 5.25 × 10−4 M Hg(NO3)2(aq) to reach the end point?
S4.5.4
9.6 × 10−3 M Cl−
Q4.5.5
Potatoes can be peeled commercially by soaking them in a 3-M to 6-M solution of sodium hydroxide, then removing the loosened skins by spraying them with water. Does a sodium hydroxide solution have a suitable concentration if titration of 12.00 mL of the solution requires 30.6 mL of 1.65 M HCI to reach the end point?
Q4.5.6
A sample of gallium bromide, GaBr2, weighing 0.165 g was dissolved in water and treated with silver nitrate, AgNO3, resulting in the precipitation of 0.299 g AgBr. Use these data to compute the %Ga (by mass) GaBr2.
S4.5.6
22.4%
Q4.5.7
The principal component of mothballs is naphthalene, a compound with a molecular mass of about 130 amu, containing only carbon and hydrogen. A 3.000-mg sample of naphthalene burns to give 10.3 mg of CO2. Determine its empirical and molecular formulas.
Q4.5.8
A 0.025-g sample of a compound composed of boron and hydrogen, with a molecular mass of ~28 amu, burns spontaneously when exposed to air, producing 0.063 g of B2O3. What are the empirical and molecular formulas of the compound.
S4.5.8
The empirical formula is BH3. The molecular formula is B2H6.
Q4.5.9
Sodium bicarbonate (baking soda), NaHCO3, can be purified by dissolving it in hot water (60 °C), filtering to remove insoluble impurities, cooling to 0 °C to precipitate solid NaHCO3, and then filtering to remove the solid, leaving soluble impurities in solution. Any NaHCO3 that remains in solution is not recovered. The solubility of NaHCO3 in hot water of 60 °C is 164 g L. Its solubility in cold water of 0 °C is 69 g/L. What is the percent yield of NaHCO3 when it is purified by this method?
Q4.5.10
What volume of 0.600 M HCl is required to react completely with 2.50 g of sodium hydrogen carbonate?
\[\ce{NaHCO3}(aq)+\ce{HCl}(aq)\rightarrow \ce{NaCl}(aq)+\ce{CO2}(g)+\ce{H2O}(l)\]
S4.5.10
49.6 mL
Q4.5.11
What volume of 0.08892 M HNO3 is required to react completely with 0.2352 g of potassium hydrogen phosphate?
\[\ce{2HNO3}(aq)+\ce{K2HPO4}(aq)\rightarrow \ce{H2PO4}(aq)+\ce{2KNO3}(aq)\]
Q4.5.12
What volume of a 0.3300-M solution of sodium hydroxide would be required to titrate 15.00 mL of 0.1500 M oxalic acid?
\[\ce{C2O4H2}(aq)+\ce{2NaOH}(aq)\rightarrow \ce{Na2C2O4}(aq)+\ce{2H2O}(l)\]
S4.5.12
13.64 mL
Q4.5.13
What volume of a 0.00945-M solution of potassium hydroxide would be required to titrate 50.00 mL of a sample of acid rain with a H2SO4 concentration of 1.23 × 10−4 M.
\[\ce{H2SO4}(aq)+\ce{2KOH}(aq)\rightarrow \ce{K2SO4}(aq)+\ce{2H2O}(l)\]
S4.5.13
1.30 mL
Q4.5.14
A sample of solid calcium hydroxide, Ca(OH)2, is allowed to stand in water until a saturated solution is formed. A titration of 75.00 mL of this solution with 5.00 × 10−2 M HCl requires 36.6 mL of the acid to reach the end point.
\[\ce{Ca(OH)2}(aq)+\ce{2HCl}(aq)\rightarrow \ce{CaCl2}(aq)+\ce{2H2O}(l)\]
What is the molarity?
S4.5.14
1.22 M
Q4.5.15
What mass of Ca(OH)2 will react with 25.0 g of propionic acid to form the preservative calcium propionate according to the equation?
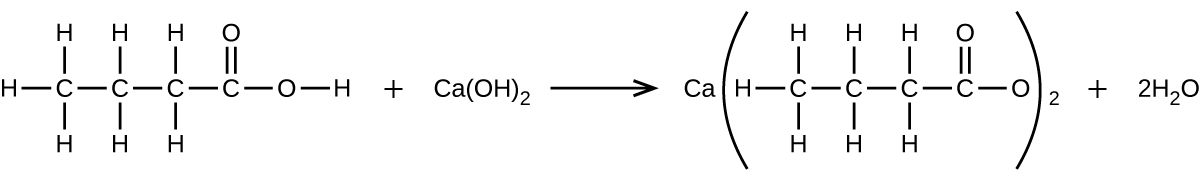
Q4.5.16
How many milliliters of a 0.1500-M solution of KOH will be required to titrate 40.00 mL of a 0.0656-M solution of H3PO4?
\[\ce{H3PO4}(aq)+\ce{2KOH}(aq)\rightarrow \ce{K2HPO4}(aq)+\ce{2H2O}(l)\]
S4.5.16
34.99 mL KOH
Q4.5.17
Potassium acid phthalate, KHC6H4O4, or KHP, is used in many laboratories, including general chemistry laboratories, to standardize solutions of base. KHP is one of only a few stable solid acids that can be dried by warming and weighed. A 0.3420-g sample of KHC6H4O4 reacts with 35.73 mL of a NaOH solution in a titration. What is the molar concentration of the NaOH?
\[\ce{KHC6H4O4}(aq)+\ce{NaOH}(aq)\rightarrow \ce{KNaC6H4O4}(aq)+\ce{H2O}(aq)\]
Q4.5.18
The reaction of WCl6 with Al at ~400 °C gives black crystals of a compound containing only tungsten and chlorine. A sample of this compound, when reduced with hydrogen, gives 0.2232 g of tungsten metal and hydrogen chloride, which is absorbed in water. Titration of the hydrochloric acid thus produced requires 46.2 mL of 0.1051 M NaOH to reach the end point. What is the empirical formula of the black tungsten chloride?
S4.5.19
The empirical formula is WCl4.


