3.7: Summary and Review Exercises
- Page ID
- 414890
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)We opened this chapter by posing the question: why do molecules exist? The short answer is that atoms, with the notable exception of certain gases such as helium, possess less potential energy when they are part of molecules than they do when they are isolated. Chemical bonds do not form unless it is energetically favorable to do so. Because change in the universe is usually directed toward minimizing potential energy, many molecules will form spontaneously. Hydrogen molecules, H2, will form upon collision of two hydrogen atoms, for example.
However, while individual atoms of a given element will usually decrease their potential energy by forming molecules such as H2 and O2, the resulting arrangement of electrons and protons is usually higher than that which can be achieved if atoms of different elements combine to form compounds. We saw this in the example of H2 and O2 reacting to form H2O and thereby decreased their potential energy. Moreover, molecules such as water can react with other molecules if it further decreases the overall potential energy. This is the trick that Nature and chemists seek to exploit when making new molecules. The correct combination of starting compounds can result in predictable, and useful, materials. Cells make ATP to supply energy to drive processes that define life, but the production of that ATP is made possible only by arranging starting molecules such that they will decrease their potential energy by so reacting. The same is true of molecules such as linoleic acid, which we discussed in Chapter 1. One of the miracles of biochemistry is the ability of cells to take simple starting materials and construct, with absolute precision, such molecules. As we develop these ideas more fully and introduce you to the logic of chemical syntheses, both biological and synthetic, you will begin to appreciate the richness of synthetic possibilities that arise from a remarkably limited selection of reaction strategies.
We will return to the topic of energy at several points in this text. When we do, we'll refine some of the definitions a bit and add some nuance, especially as energy relates to work and how energy transformations relate to processes being either spontaneous or nonspontaneous. Our decision to delay some of those ideas is intended to give you important conceptual insights about energy early in your study of chemistry without getting bogged down too much in some of the finer points. Energy is, after all, at the core of all chemical change so the sooner you begin to get comfortable with the topic, the better prepared you will be to deepen your understanding when it becomes necessary.
Review Problems.
3.12) Technetium (Tc) is an "artificial element", meaning that it is not found in naturally but it is generated by humans via nuclear processes. All known isotopes of technetium are radioactive. These include technetium-95, technetium-96, technetium-97, technetium-98, and (you guessed it!) technetium-99. How many protons and neutrons are in the nuclei of these isotopes of technetium? Why is it not possible to state how many electrons are in these isotopes, at least without more clarification in the wording of the question?
3.13) Magnesium (Mg) and manganese (Mn) are two elements that are occasionally confused for one another. They are both metals but they have very different reaction patterns. Magnesium is usually only exists as the metallic element (pure magnesium) or in the form of ions with a +2 charge (Mg2+). Manganese, in contrast, can exist as in the metallic form, or as with charges ranging from -1 to +7. How many protons and electrons are in neutral atoms of these two elements and in the following ions: Mg2+, Mn2+, Mn-, Mn4+, and Mn7+? Can you also state how many neutrons are in these ions? Why or why not?
3.14) In the text we discussed the possibility of splitting a gold atom. If you were to do so, what you would end up with is not gold. But what is it? Speculate what you might get by splitting an atom of gold-197 into two particles of roughly the same size. Based on your understanding of nuclear stability, do you think these new atoms will be stable?
3.15) Consider a scenario in which two positively charged particles approach one another. Make a sketch of the potential energy as a function of the distance between the particles. In a few sentences, explain the reasoning behind your sketch.

3.16) In the 1600s Robert Fludd, a prominent English physician who also had interests in mathematics and philosophy, designed the machine, illustrated at left. His "water screw machine" uses an elevated reservoir of water as an energy source. Water flows out and turns a wheel which, in turn, drives a shaft that does several things: it turns a millstone (to grind grain, for example) and it turns a grooved screw that carries water back up to the reservoir. The water is then available to drive the wheel again. Comment on the feasibility of such a device, referencing the Laws of Thermodynamics as applicable.
(Image is in the Public Domain, https://commons.wikimedia.org/w/inde...?curid=2854454
3.17) Ethanol (C2H5OH) is a commonly used additive for gasoline. Estimate energy change for burning one mole ethanol. In your calculations, don't forget that CO2 has two C=O double bonds, and that oxygen gas (O2) has one O=O bond.
3.18) Table sugar is virtually pure sucrose (structure shown at right). The nutrition label on a package of table sugar s tates that one serving (8 grams) provides 30 Calories. Use the bond energies in Table 3-2 to check this value by assuming sugar is completely "combusted" via the following reaction. Recall that 1 Calorie = 1000 calories and 1 calories = 4.18 J. How well does your calculation agree with the label? In your calculations, don't forget that CO2 has two C=O double bonds, and that oxygen gas (O2) has one O=O bond.
tates that one serving (8 grams) provides 30 Calories. Use the bond energies in Table 3-2 to check this value by assuming sugar is completely "combusted" via the following reaction. Recall that 1 Calorie = 1000 calories and 1 calories = 4.18 J. How well does your calculation agree with the label? In your calculations, don't forget that CO2 has two C=O double bonds, and that oxygen gas (O2) has one O=O bond.
\[ \ce{C12H22O11 + \frac{35}{2} O2 -> 11 H2O + 12 CO2} \nonumber \]
3.19) The energy content of fuels is often expressed in terms of kJ per gram, rather than on a molar basis, such as kJ per mole. Calculate the heat content of H2, octane, and wood in kJ per gram and suggest why using a mass basis instead of a molar basis is so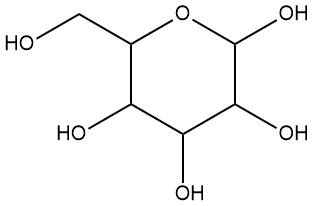 metimes preferable. To estimate the heat content of wood make the following simplifying assumption: assume it is made from glucose (C6H12O6, structure at right), the sugar that is made via photosynthesis. Is this a reasonable assumption? Wood is primarily cellulose, which is based on glucose so it serves as a reasonable approximation, but because the reaction that produces cellulose also produces water as a product, there will be a slight error in your result (it will be a bit less exothermic than you estimate). In your calculations, don't forget that CO2 has two C=O double bonds, and that oxygen gas (O2) has one O=O bond.
metimes preferable. To estimate the heat content of wood make the following simplifying assumption: assume it is made from glucose (C6H12O6, structure at right), the sugar that is made via photosynthesis. Is this a reasonable assumption? Wood is primarily cellulose, which is based on glucose so it serves as a reasonable approximation, but because the reaction that produces cellulose also produces water as a product, there will be a slight error in your result (it will be a bit less exothermic than you estimate). In your calculations, don't forget that CO2 has two C=O double bonds, and that oxygen gas (O2) has one O=O bond.
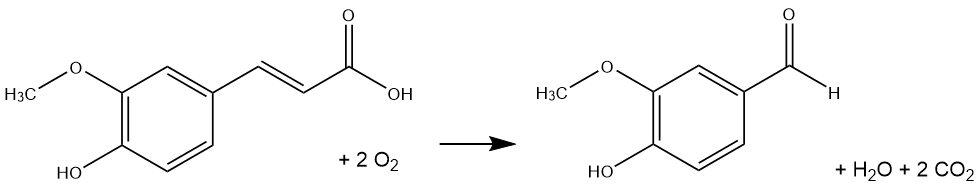
3.20) Estimate the energy change for the fermentation of ferulic acid to vanillin, shown in the equation below. In your calculations, don't forget that CO2 has two C=O double bonds, and that oxygen gas (O2) has one O=O bond.
 3.21) The main component of olive oil is oleic acid (structure shown at right). If a serving size is one tablespoon (15 g), estimate how many Calories are provided by one serving of olive oil, assuming that the minor components have the same energy content. In other words, assume that olive oil is pure oleic acid. In your calculations, don't forget that CO2 has two C=O double bonds, and that oxygen gas (O2) has one O=O bond.
3.21) The main component of olive oil is oleic acid (structure shown at right). If a serving size is one tablespoon (15 g), estimate how many Calories are provided by one serving of olive oil, assuming that the minor components have the same energy content. In other words, assume that olive oil is pure oleic acid. In your calculations, don't forget that CO2 has two C=O double bonds, and that oxygen gas (O2) has one O=O bond.

