24.2 Structure and Properties of Amines
- Page ID
- 91030
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Objectives
After completing this section, you should be able to
- describe the geometry and bonding of simple amines.
- explain why most chiral amines cannot be resolved into their two enantiomers.
Make certain that you can define, and use in context, the key term below.
- pyramidal inversion
Molecular models may help you to understand pyramidal inversion.
Amines typically have three bonds and one pair of lone pair electrons. This makes the nitrogen sp3 hybridized, trigonal pyramidal, with a bond angle of roughly 109.5o.
Stereogenic Nitrogen
Single-bonded nitrogen is pyramidal in shape, with the non-bonding electron pair pointing to the unoccupied corner of a tetrahedral region. Since the nitrogen in these compounds is bonded to three different groups, its configuration is chiral. The non-identical mirror-image configurations are illustrated in the following diagram (the remainder of the molecule is represented by R, and the electron pair is colored yellow). If these configurations were stable, there would be four additional stereoisomers of ephedrine and pseudoephedrine. However, pyramidal nitrogen is normally not configurationally stable. It rapidly inverts its configuration (equilibrium arrows) by passing through a planar, sp2-hybridized transition state, leading to a mixture of interconverting R and S configurations. If the nitrogen atom were the only chiral center in the molecule, a 50:50 (racemic) mixture of R and S configurations would exist at equilibrium. If other chiral centers are present, as in the ephedrin isomers, a mixture of diastereomers will result. The take-home message is that nitrogen does not contribute to isolable stereoisomers.
Boiling Point and Water Solubility
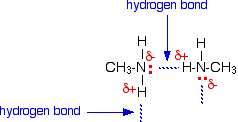
It is instructive to compare the boiling points and water solubility of amines with those of corresponding alcohols and ethers. The dominant factor here is hydrogen bonding, and the first table below documents the powerful intermolecular attraction that results from -O-H---O- hydrogen bonding in alcohols (light blue columns). Corresponding -N-H---N- hydrogen bonding is weaker, as the lower boiling points of similarly sized amines (light green columns) demonstrate. Alkanes provide reference compounds in which hydrogen bonding is not possible, and the increase in boiling point for equivalent 1º-amines is roughly half the increase observed for equivalent alcohols.
| Compound | CH3CH3 | CH3OH | CH3NH2 | CH3CH2CH3 | CH3CH2OH | CH3CH2NH2 |
|---|---|---|---|---|---|---|
| Mol.Wt. | 30 | 32 | 31 | 44 | 46 | 45 |
| Boiling Point ºC |
-88.6º | 65º | -6.0º | -42º | 78.5º | 16.6º |
The second table illustrates differences associated with isomeric 1º, 2º & 3º-amines, as well as the influence of chain branching. Since 1º-amines have two hydrogens available for hydrogen bonding, we expect them to have higher boiling points than isomeric 2º-amines, which in turn should boil higher than isomeric 3º-amines (no hydrogen bonding). Indeed, 3º-amines have boiling points similar to equivalent sized ethers; and in all but the smallest compounds, corresponding ethers, 3º-amines and alkanes have similar boiling points. In the examples shown here, it is further demonstrated that chain branching reduces boiling points by 10 to 15 ºC.
| Compound | CH3(CH2)2CH3 | CH3(CH2)2OH | CH3(CH2)2NH2 | CH3CH2NHCH3 | (CH3)3CH | (CH3)2CHOH | (CH3)2CHNH2 | (CH3)3N |
|---|---|---|---|---|---|---|---|---|
| Mol.Wt. | 58 | 60 | 59 | 59 | 58 | 60 | 59 | 59 |
| Boiling Point ºC |
-0.5º | 97º | 48º | 37º | -12º | 82º | 34º | 3º |
The water solubility of 1º and 2º-amines is similar to that of comparable alcohols. As expected, the water solubility of 3º-amines and ethers is also similar. These comparisons, however, are valid only for pure compounds in neutral water. The basicity of amines (next section) allows them to be dissolved in dilute mineral acid solutions, and this property facilitates their separation from neutral compounds such as alcohols and hydrocarbons by partitioning between the phases of non-miscible solvents.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

