23.12 The Robinson Annulation Reaction
- Page ID
- 91025
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Objectives
After completing this section, you should be able to
- write an equation to illustrate the Robinson annulation reaction.
- identify the cyclic product formed when a 1,5‑diketone is treated with base.
- identify the carbonyl compounds and any other reagents needed to synthesize a given cyclic compound by a series of reactions, one of which is a Robinson annulation.
- Robinson annulation reaction
The building of an alicyclic compound from acyclic starting materials can present an interesting challenge to the synthetic organic chemist. One route by which such a synthesis can be achieved is through the use of the Robinson annulation reaction. This reaction, which may at first look very complex, can be readily understood once you realize that it simply involves a Michael reaction followed by an intramolecular aldol condensation. Both of these steps involve attack by enolate anions.
As in some of the other syntheses that you have studied, when you are simply given the structure of a compound and asked how it could have been prepared, it can be difficult to recognize which reactions might have been used. In this instance, keep in mind that you have studied relatively few reactions which have resulted in the formation of an alicyclic compound. Thus, when asked how such a compound might be prepared, you should keep the possibility of using a Robinson annulation reaction in mind.
Many times the product of a Michael addition produces a dicarbonyl which can then undergo an intramolecular aldol reaction. These two processes together in one reaction creates two new carbon-carbon bonds and also creates a ring. Ring-forming reactions are called annulations after the Latin work for ring annulus. The reaction is named after English chemist Sir Robert Robinson (1886-1975) who developed it. He received the Nobel prize in chemistry in 1947. Remember that during annulations five and six membered rings are preferred.
Examples of Robinson Annulations
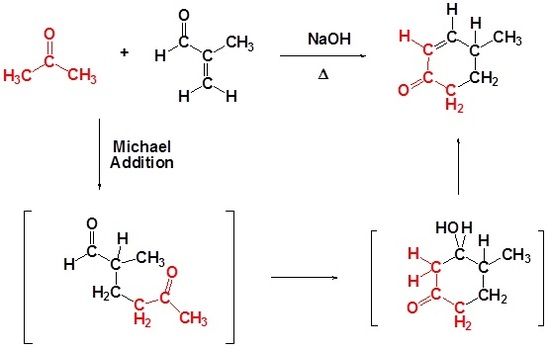
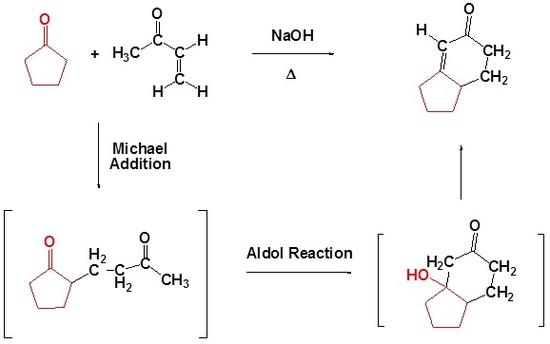
Draw the product of the following Robinson Annulation.
- Answer
-
Analysis: When considering the product of a Robinson annulation it is usually best to consider each reaction individually. Use the steps discussed in Section 23.10 to convert the starting materials into the product of a Michael reaction, then into the product of an intramolecular aldol condensation. If the starting materials are drawn in a skeletal structure it may be helpful to convert to a condensed formula to better keep track of carbons and hydrogens.
Formation of the Michael reaction product
Formation of the intramolecular Aldol condensation product
Exercises
Provide products of the following Robinson annulations.
a)
b)
c)
- Answer
-
a)
b)
c)
What would be the starting materials required to make the following molecule using a Robinson annulation?
- Answer
-
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)

