21.10 Spectroscopy of Carboxylic Acid Derivatives
- Page ID
- 91001
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Objectives
After completing this section, you should be able to
- identify the region of the infrared spectrum in which absorptions resulting from the carbonyl group of carboxylic acid derivatives occur.
- identify the characteristic features of the infrared spectra of acid anhydrides and nitriles that allow us to use infrared spectroscopy to distinguish between these compounds and the others discussed in this unit.
- use a table of characteristic infrared absorptions to interpret the infrared spectra of carbonyl‑containing compounds.
- use infrared spectroscopy and 1H NMR spectroscopy in the deduction of the structure of an unknown compound.
Note: You should recognize that protons on carbon atoms next to carbonyl groups are slightly deshielded, and absorb near 2 δ in the 1H NMR spectrum.
The influence of heteroatom substituents on the reactivity of carbonyl functions toward nucleophiles was discussed earlier with respect to carboxylic acid derivatives. A useful relationship exists between the reactivity of these derivatives and their carbonyl stretching frequencies. Thus, the very reactive acyl halides and anhydrides absorb at frequencies significantly higher than ketones, whereas the relatively unreactive amides absorb at lower frequencies. These characteristics are listed below.
Infrared spectra of many carboxylic acid derivatives will be displayed in the figure below the table by clicking the appropriate buttons presented there.
|
Carbonyl Derivative |
Carbonyl Absorption |
Comments |
|---|---|---|
|
Acyl Halides (RCOX) |
C=O stretch |
Conjugation lowers the C=O frequencies reported here, as with aldehydes & ketones. In acyl chlorides a lower intensity shoulder or peak near 1740 cm-1 is due to an overtone interaction. |
|
Acid Anhydride, (RCO)2O |
C=O stretch (2 bands) |
Conjugation lowers the C=O frequencies reported here, as with aldehydes & ketones. The two stretching bands are separated by 60 ± 30 cm-1, and for acyclic anhydrides the higher frequency (asymmetric stretching) band is stronger than the lower frequency (symmetric) absorption. Cyclic anhydrides also display two carbonyl stretching absorptions, but the lower frequency band is the strongest. One or two -CO-O-CO- stretching bands are observed in the 1000 to 1300 cm-1 region. |
|
Esters & Lactones (RCOOR') |
C=O stretch |
Conjugation lowers the C=O frequencies reported here, as with aldehydes & ketones Strong CO-O stretching absorptions (one ot two) are found from 1150 to 1250 cm-1 |
|
Amides & Lactams (RCONR2) |
C=O bands |
The effect of conjugation is much less than for aldehydes & ketones. The higher frequency absorption (1665± 30) is called the Amide I band. The lower frequency Amide II band (1620± 30 in 1° amides & 1530± 30 in 2° amides) is largely due to N-H bending trans to the carbonyl oxygen. In concentrated samples this absorption is often obscured by the stronger amide I absorption. Hydrogen bonded association shifts some of these absorptions, as well as the prominent N-H stretching absorptions. N-H stretch: 3170 to 3500 cm-1. Two bands for 1°-amides, one for 2°-amides. |
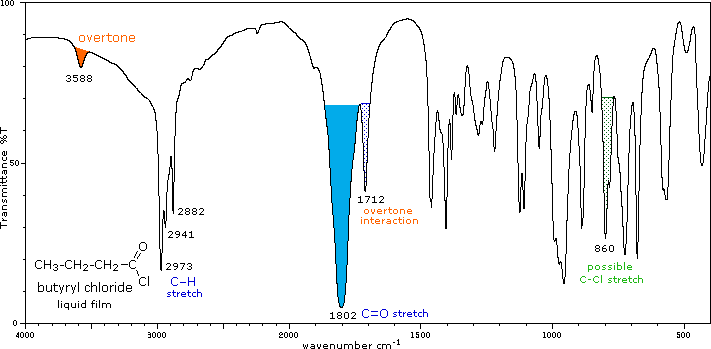
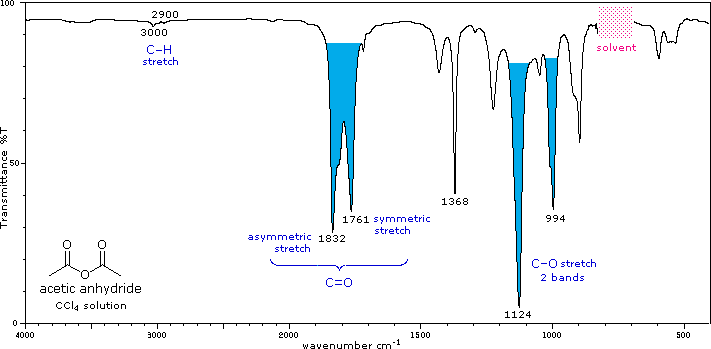
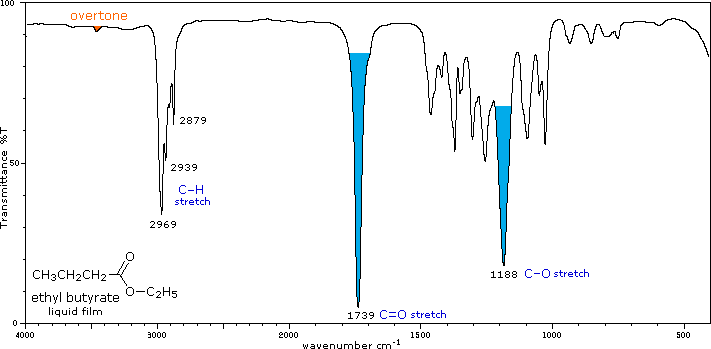
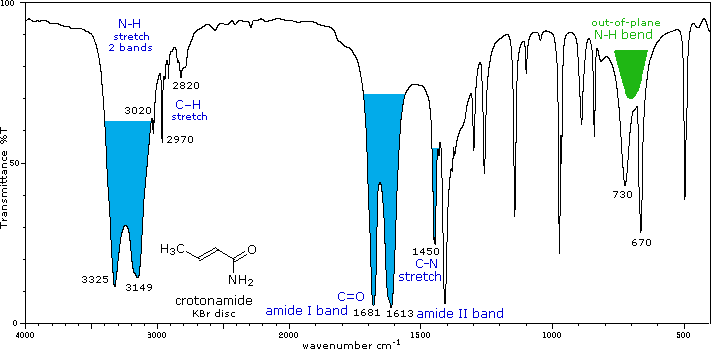
1H NMR Spectra
Protons on carbons adjacent to carbonyls absorb at ~2.0-2.5 ppm.
The N-H protons attached to primary and secondary amines absorb at ~7.5-8.5 ppm.
Resonances from protons directly attached to the carbon bonded to nitrogen in an amide tend to resonate in the 2.0-3.0 ppm region of an NMR spectra. The NH protons of primary and secondary amides resonate in the 5.5-8.5 ppm regions. Like other hydrogen bonding protons, these NH proton peaks appear broad (14N quadrapole broadening) and can be washed out by the hydrogen-deuterium exchange created by D2O addition.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Jim Clark (Chemguide.co.uk)

