19.6 Nucleophilic Addition of HCN: Cyanohydrin Formation
- Page ID
- 90969
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Objectives
After completing this section, you should be able to
- write an equation to describe the formation of a cyanohydrin from an aldehyde or ketone.
- identify the cyanohydrin formed from the reaction of a given aldehyde or ketone with hydrogen cyanide.
- identify the aldehyde or ketone, the reagents, or both, needed to prepare a given cyanohydrin.
- write the detailed mechanism for the addition of hydrogen cyanide to an aldehyde or ketone.
- write equations to represent the conversion of cyanohydrins to hydroxyamines and hydroxycarboxylic acids, and hence recognize the importance of cyanohydrin formation in organic synthesis.
a. identify the product formed when a given cyanohydrin is either reduced with lithium aluminum hydride or hydrolyzed with mineral acid.
b. identify the aldehyde or ketone, and other reagents needed to prepare a given α‑hydroxyamine or α‑hydroxycarboxylic acid.
Make certain that you can define, and use in context, the key term below.
- cyanohydrin
For successful cyanohydrin formation it is important to have free cyanide ions available to react with the ketone or aldehyde. This can be achieved by using a salt (e.g. KCN or NaCN) or a silylated (e.g. Me3SiCN) form of cyanide under acidic conditions or by using HCN with some base added to produce the needed CN− nucleophile.
Cyanohydrins have the structural formula of R2C(OH)CN. The “R” on the formula represents an alkyl, aryl, or hydrogen. To form a cyanohydrin, a hydrogen cyanide adds reversibly to the carbonyl group of an organic compound thus forming a hydroxyalkanenitrile adducts (commonly known and called as cyanohydrins).
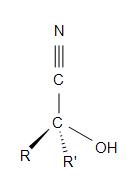
Figure 19.6.1: General structure of a cyanohydrin
Mechanism of Cyanohydrin Formation
In the first step of the mechanism, the cyanide ion acts as a nucleophile and forms a C-C bond with the electrophillic carbonyl carbon. The two electrons in the carbonyl pi bond are pushed on to the electronegative oxygen forming a tetrahedral alkoxide ion intermediate. In the second step, the alkoxide ion is protonated by HCN which regenerates the cyanide ion.
Step 1: Nucleophilic attack
Step 2: Protonation
Acetone Cyanohydrins
Acetone cyanohydrins (ACH) have the structural formula of (CH3)2C(OH)CN. It is an organic compound serves in the production of methyl methacrylate (also known as acrylic).

Figure 19.6.2: Acetone cyanohydrins
It is classified as an extremely hazardous substance, since it rapidly decomposes when it's in contact with water. In ACH, sulfuric acid is treated to give the sulfate ester of the methacrylamid. Preparations of other cyanohydrins are also used from ACH: for HACN to Michael acceptors and for the formylation of arenas. The treatment with lithium hydride affords anhydrous lithium cyanide.
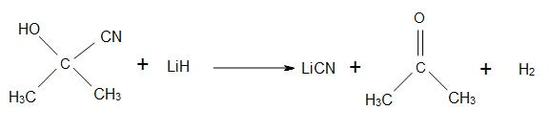
Figure 19.6.3: Reduction of Acetone cyanohydrins
Further Chemistry of Cyanohydrins
Cyanohydrin functional groups often prove useful because of the further chemistry that can be carried out due to the presence of a hydroxyl and a nitrile functionality. In particular, dehydration can convert the hydroxyl group into an alkene (Section 17.6). The nitrile can be converted into a carboxylic acid function group through reaction with a hot acidic aqueous solution (Section 20.7). Also, the nitrile can be reduced by the addition of LiAlH4 to form a primary amine.
Other Cyanohydrins
Other interesting cyanohydrins are: acetone cyanohydrin, and glycolonitrile.
Acetone cyanohydrin has the structure, (CH3)2C(OH)CN, and is used in the production of methyl methacrylate (also known as acrylic). Glycolonitrile is an organic compound with the structural formula of HOCH2CN, which is the simplest cyanohydrin that is derived by formaldehydes.
Exercise
What product is formed in this reaction?
- Answer
-
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Jim Clark (Chemguide.co.uk)

