3.3: Alkyl Groups
- Page ID
- 67069
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- recognize and name any alkyl group that can be considered to have been formed by the removal of a terminal hydrogen atom from a straight-chain alkane containing ten or fewer carbon atoms.
- explain what is meant by a primary, secondary, tertiary or quaternary carbon atom.
- represent the various types of organic compounds using the symbol “R” to represent any alkyl group.
Make certain that you can define, and use in context, the key terms below.
- alkyl group
- methyl group
- primary carbon
- quaternary carbon
- secondary carbon
- tertiary carbon
The differences among primary, secondary, tertiary and quaternary carbon atoms are explained in the following discussion. A convenient way of memorizing this classification scheme is to remember that a primary carbon atom is attached directly to only one other carbon atom, a secondary carbon atom is attached directly to two carbon atoms, and so on.
The IUPAC system requires first that we have names for simple unbranched chains, as noted above, and second that we have names for simple alkyl groups that may be attached to the chains. Examples of some common alkyl groups are given in the following table. Note that the "ane" suffix is replaced by "yl" in naming groups. The symbol R is used to designate a generic (unspecified) alkyl group.
| Group | CH3– | C2H5– | CH3CH2CH2– | (CH3)2CH– | CH3CH2CH2CH2– | (CH3)2CHCH2– | CH3CH2CH(CH3)– | (CH3)3C– | R– |
|---|---|---|---|---|---|---|---|---|---|
| Name | Methyl | Ethyl | Propyl | Isopropyl | Butyl | Isobutyl | sec-Butyl | tert-Butyl | Alkyl |
Alkyl Groups
Alkanes can be described by the general formula CnH2n+2. An alkyl group is formed by removing one hydrogen from the alkane chain and is described by the formula CnH2n+1. The removal of this hydrogen results in a stem change from -ane to -yl. Take a look at the following examples.
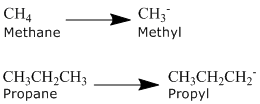
The same concept can be applied to any of the straight chain alkane names provided in Table \(\PageIndex{2}\).
| Name | Molecular Formula | Condensed Structural Formula |
|---|---|---|
| Methane | CH4 | CH4 |
| Ethane | C2H6 | CH3CH3 |
| Propane | C3H8 | CH3CH2CH3 |
| Butane | C4H10 | CH3(CH2)2CH3 |
| Pentane | C5H12 | CH3(CH2)3CH3 |
| Hexane | C6H14 | CH3(CH2)4CH3 |
| Heptane | C7H16 | CH3(CH2)5CH3 |
| Octane | C8H18 | CH3(CH2)6CH3 |
| Nonane | C9H20 | CH3(CH2)7CH3 |
| Decane | C10H22 | CH3(CH2)8CH3 |
| Undecane | C11H24 | CH3(CH2)9CH3 |
| Dodecane | C12H26 | CH3(CH2)10CH3 |
| Tridecane | C13H28 | CH3(CH2)11CH3 |
| Tetradecane | C14H30 | CH3(CH2)12CH3 |
| Pentadecane | C15H32 | CH3(CH2)13CH3 |
| Hexadecane | C16H34 | CH3(CH2)14CH3 |
| Heptadecane | C17H36 | CH3(CH2)15CH3 |
| Octadecane | C18H38 | CH3(CH2)16CH3 |
| Nonadecane | C19H40 | CH3(CH2)17CH3 |
| Eicosane | C20H42 | CH3(CH2)18CH3 |
Classification of carbon atoms
Carbons have a special terminology to describe how many other carbons they are attached to.
- Primary carbons (1o) attached to one other C atom
- Secondary carbons (2o) are attached to two other C’s
- Tertiary carbons (3o) are attached to three other C’s
- Quaternary carbons (4o) are attached to four C's
You will find that hydrogen atoms are also classified in this manner. A hydrogen atom attached to a primary carbon atom is called a primary hydrogen; thus, isobutane, has nine primary hydrogens and one tertiary hydrogen.
- Primary hydrogens (1o) are attached to carbons bonded to one other C atom
- Secondary hydrogens (2o) are attached to carbons bonded to two other C’s
- Tertiary hydrogens (3o) are attached to carbons bonded to three other C’s
Exercises
Determine whether the H’s indicated in the following structure are 1o, 2o, or 3o.
- Answer
-
Determine whether the H’s indicated in the following structure is 1o, 2o, or 3o.
- Answer
-
Determine whether the carbons indicated in the structure below are 1o, 2o, 3o, or 4o.
- Answer
-
Determine whether the carbons indicated in the structure below are 1o, 2o, 3o, or 4o.
- Answer
-
Please indicate the total number of each type 1o, 2o, 3o, and 4o carbons in the following molecule.
- Answer
-
There are 5 primary (1o) C’s, 2 secondary (2o) C’s, 1 tertiary (3o) C and 1 quaternary (4o) C in the structure (seen color coded below).
Please indicate the total number of each type 1o, 2o, 3o, and 4o carbons in the following molecule.
- Answer
-
There are 8 primary (1o) C’s, 7 secondary (2o) C’s, 2 tertiary (3o) C’s and 2 quaternary (4o) C’s in the structure (seen color coded below).
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)

