15.2: Common Acids and Their Uses
- Page ID
- 358669
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)⚙️ Learning Objectives
- Identify sources and uses of common acids.
You are likely familiar with a number of common acids and their uses. Several common acids and their uses are described below.
Hydrochloric Acid
Hydrochloric acid is a corrosive, strong mineral acid with many industrial uses. It is a colorless, highly pungent solution of hydrogen chloride gas, HCl (g), in water. Hydrochloric acid is usually prepared by treating HCl (g) with water.
\(\mathrm{HCl}\;(g)\;\xrightarrow{{\mathrm H}_2\mathrm O}\mathrm{HCl}\;(aq)\)
Hydrochloric acid is a strong acid, since it is completely dissociated in water. Hydrochloric acid is the preferred acid in titration for determining the amount of bases and can also be used to prepare various chloride salts. It is often sold under the common name of muriatic acid for applications like cleaning concrete and pools.
Sulfuric Acid
Sulfuric acid is a highly corrosive strong mineral acid with the molecular formula H2SO4. Sulfuric acid has a wide range of applications including use in domestic acidic drain cleaners, as an electrolyte in lead-acid batteries, and in various cleaning agents. It is also a central substance in the chemical industry.
Sulfuric acid behaves as a typical acid in its reaction with most metals by generating hydrogen gas. Because the hydration of sulfuric acid is thermodynamically favorable (and is highly exothermic) and the affinity of it for water is sufficiently strong, sulfuric acid is an excellent dehydrating agent. Concentrated sulfuric acid has a very powerful dehydrating property, removing water from other compounds including sugar and other carbohydrates and producing carbon, heat, steam (see Video \(\PageIndex{1}\)).
\({\mathrm C}_{12}{\mathrm H}_{22}{\mathrm O}_{11}\;(s)\;\xrightarrow{{\mathrm H}_2{\mathrm{SO}}_4}\;12\;\mathrm C\;(s)\;+\;11\;{\mathrm H}_2\mathrm O\;(g)\)
Nitric Acid
Nitric acid, HNO3, is a highly corrosive mineral acid and is also commonly used as a strong oxidizing agent. Nitric acid is normally considered to be a strong acid at ambient temperatures. Nitric acid can be made by reacting nitrogen dioxide, NO2, with water.
3 NO2 (g) + H2O (l) → 2 HNO3 (aq) + NO (g)
Nitric acid reacts with most metals, but the details depend on the concentration of the acid and the nature of the metal. Dilute nitric acid behaves as a typical acid in its reaction with most metals. For example, magnesium, manganese, and zinc liberate hydrogen gas:
Mg (s) + 2 HNO3 (aq) → Mg(NO3)2 (aq) + H2 (g)
Mn (s) + 2 HNO3 (aq) → Mn(NO3)2 (aq) + H2 (g)
Zn (s) + 2 HNO3 (aq) → Zn(NO3)2 (aq) + H2 (g)
However, concentrated nitric acid reacts with copper metal in a much more interesting manner, yielding the reddish-brown gas nitrogen dioxide, NO2, as one of the products.
Cu (s) + 4 HNO3 (aq) → Cu(NO3)2 (aq) + 2 NO2 (g) + 2 H2O (l)
⛓ Nitric Acid Acts Upon Copper
Nitric acid is a corrosive acid and a powerful oxidizing agent. The major hazard it poses is chemical burns, as it carries out acid hydrolysis with proteins (amides) and fats (esters) which consequently decomposes living tissue (Figure \(\PageIndex{1}\)). Concentrated nitric acid stains human skin yellow due to its reaction with the keratin.
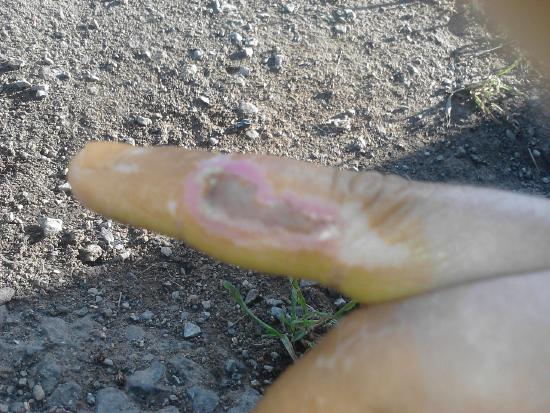
The following excerpt is taken from the biographical memoir of Ira Remsen (1846-1927), a notable chemistry educator and founder of chemical journals, and was presented to the National Academy of Sciences at the annual meeting in 1931:
"While reading a text-book of chemistry I came upon the statement, 'nitric acid acts upon copper'. I was getting tired of reading such absurd stuff and I determined to see what this meant. Copper was more or less familiar to me, for copper cents were then in use. I had seen a bottle marked 'nitric acid' on a table in the doctor's office where I was then 'doing time!' I did not know its peculiarities but I was getting on and likely to learn. The spirit of adventure was upon me.
"Having nitric acid and copper, I had only to learn what the words 'acts upon' meant. Then the statement, 'nitric acid acts upon copper' would be something more than mere words. All was still. In the interest of knowledge I was even willing to sacrifice one of the few copper cents then in my possession.
"I put one of them on the table; opened the bottle marked 'nitric acid'; poured some of the liquid on the copper; and prepared to take an observation. But what was this wonderful thing I beheld ? The cent was already changed, and it was no small change either. A greenish blue liquid foamed and fumed over the cent and over the table. The air in the neighborhood of the performance became colored dark red. A great colored cloud arose. This was disagreeable and suffocating. How should I stop this?
"I tried to get rid of the objectionable mess by picking it up and throwing it out of the window, which I had meanwhile opened. I learnt another fact—nitric acid not only acts on copper but it acts on fingers. The pain led to another unpremeditated experiment. I drew my fingers across my trousers and another fact was discovered. Nitric acid acts upon trousers.
"Taking everything into consideration, that was the most impressive experiment, and, relatively, probably the most costly I have ever performed. I tell it even now with interest. It was a revelation to me. It resulted in a desire on my part to learn more about that kind of action. Plainly the only way to learn about it was to see its results, to experiment, to work in a laboratory."
What does this reaction look like? Check out Video \(\PageIndex{2}\) to take a peek!
Carbonic Acid
Carbonic acid is a chemical compound with the chemical formula H2CO3 and is also a name sometimes given to solutions of carbon dioxide in water (carbonated water), because such solutions contain small amounts of H2CO3 (aq). Carbonic acid, which is a weak acid, forms two kinds of salts: the carbonates and the bicarbonates. In geology, carbonic acid causes limestone to dissolve, producing calcium bicarbonate – which leads to many limestone features such as stalactites and stalagmites.
When carbon dioxide dissolves in water, it exists in chemical equilibrium, producing carbonic acid:
CO2 (g) + H2O (l) ⇄ H2CO3 (aq)
The reaction can be pushed to favor the reactants to generate CO2 (g) from solution, which is key to the bubbles observed in carbonated beverages (Figure \(\PageIndex{2}\)).

Formic Acid
Formic acid, HCOOH, is the simplest carboxylic acid and is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. The word "formic" comes from the Latin word for ant, formica, referring to its early isolation by the distillation of ant bodies. Formic acid occurs widely in nature as its conjugate base formate.
Acetic Acid
Acetic acid, HC2H3O2, is the second simplest carboxylic acid. It is the acid found in vinegar, usually at a concentration of 5% acetic acid. Organic chemists usually write the formula of acetic acid as CH3COOH to indicate the presence of the carboxyl group. The IUPAC name of acetic acid is ethanoic acid. In other words, acetic acid and ethanoic acid are the same substance.
The oxidation of ethanol, CH3CH2OH, the alcohol found in alcoholic beverages, leads to the production of acetic acid and is the reason why a bottle of wine will begin to taste sour and take on a vinegary taste several days after it has been opened and exposed to the air. Industrially, most acetic acid is produced by the carbonylation of methanol using carbon monoxide:
\({\mathrm{CH}}_3\mathrm{OH}\;+\;\mathrm{CO}\;\xrightarrow{\mathrm{catalyst}}\;{\mathrm{CH}}_3\mathrm{COOH}\)
Citric Acid
Citric acid, H3C6H5O7, is a weak organic tricarboxylic acid that occurs naturally in citrus fruits. The citrate ion is an intermediate in the TCA cycle (Krebs cycle), a central metabolic pathway for animals, plants and bacteria. Because it is one of the stronger edible acids, the dominant use of citric acid is as a flavoring and preservative in food and beverages, especially soft drinks.

Acetylsalicylic Acid
Acetylsalicylic acid (also known as aspirin) is a medication used to treat pain, fever, and inflammation. Aspirin, in the form of leaves from the willow tree, has been used for its health effects for at least 2,400 years. Aspirin is a white, crystalline, weakly acidic substance.
Summary
| Common Name | Uses | |
|---|---|---|
hydrochloric acid, HCl |
muriatic acid (used in pools) stomach acid |
Used in cleaning (refining) metals, in maintenance of swimming pools, and for household cleaning. |
sulfuric acid, H2SO4 |
Used in car batteries and in the manufacture of fertilizers. | |
nitric acid, HNO3 |
Used in the manufacture of fertilizers, explosives, and in extraction of gold. | |
acetic acid, HC2H3O2 |
vinegar | Main ingredient in vinegar. |
carbonic acid, H2CO3 |
responsible for the "fizz" in carbonated drinks | As an ingredient in carbonated drinks. |
citric acid, H3C6H5O7 |
Used in food and dietary supplements. Also added as an acidulant in creams, gels, liquids, and lotions. | |
acetylsalicylic acid, HC9H7O4 |
aspirin | The active ingredient in aspirin. |
This page is shared under a CK-12 license and was authored, remixed, and/or curated by Peggy Lawson (Oxbow Prairie Heights School), funded by Saskatchewan Educational Technology Consortium; Melissa Alviar-Agnew, Henry Agnew, Lance S. Lund (Anoka-Ramsey Community College), and Wikipedia. Original source: https://www.ck12.org/c/chemistry/.



