14.8: Colligative Properties of Solutions
- Page ID
- 289460
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)⚙️ Learning Objectives
- Explain what the term "colligative" means, and list the colligative properties.
- Indicate what happens to the boiling point and the freezing point of a solvent when a solute is added to it.
People who live in colder climates have seen trucks put salt on the roads when snow or ice is forecast (Figure \(\PageIndex{1}\)), but why is this done? It turns out that the addition of a solute lowers the freezing point of a liquid, a phenomenon called freezing point depression. Freezing point depression is one the four different kinds of colligative properties of solutions. Colligative properties are properties of solutions that depend only on the concentration of solute particles in a solvent, but not their identity. What this means for the example above is that people in colder climates do not necessarily need salt to get the same effect on the roads – any solute will work. However, the higher the concentration of solute, the more these properties will change.

The four colligative properties of solutions are:
- freezing point depression
- boiling point elevation
- vapor pressure depression (not discussed in this text)
- osmotic pressure elevation
Freezing Point Depression
The freezing point of a solution is lower than the freezing point of a pure solvent, which is why the phenomenon is called freezing point depression. The freezing point of a substance is the temperature at which the liquid changes into a solid. At a given temperature, if a substance is added to a solvent (such as water), the solute-solvent interactions prevent the solvent from transitioning into the solid phase. The solute-solvent interactions require the temperature to decrease further (slowing the particles down) in order to solidify the solution.
Salt is placed onto roads and sidewalks in the winter to prevent water from freezing at the normal temperature of 0°C and, instead, causing it to freeze at a lower temperature, sometimes as low as –10°C to –15°C. De-icing planes is another common example of freezing point depression in action. A number of solutions are used, but commonly a solution such as ethylene glycol, or a less toxic monopropylene glycol, is used to de-ice aircraft. Aircrafts are sprayed with the solution when the temperature is predicted to drop below the freezing point. Yet another example of freezing point depression is the addition of antifreeze to a car radiator. This prevents the radiator from freezing in the winter. Freezing water can cause an engine block to crack, since water expands upon freezing.
Freezing point depression is observed for any solute added to a solvent; the freezing point of the solution will always be lower than the freezing point of the pure solvent (without the solute). Thus, when anything is dissolved in water, the solution will freeze at a lower temperature than pure water.

Boiling Point Elevation
Pure water boils at 100°C at 1 atm of pressure. When table salt is added to water, the resulting solution has a higher boiling point than pure water. Since the addition of a solute increases the boiling point, the colligative property is called boiling point elevation. In the case of salt, the boiling point increases because the ions form an attraction with the water molecules that prevents them from escaping as easily into the gaseous phase. In order for a saltwater solution to boil, the temperature must be raised above 100°C at 1 atm of pressure. Boiling point elevation is observed for any nonvolatile solute (one that does not evaporate easily) that is added to a solvent; the boiling point of a solution will always be higher than the boiling point of the pure liquid.
One such application of boiling point elevation is the addition of antifreeze to a car radiator. Interestingly enough, the term antifreeze is rarely heard during the summer or in warmer climates. The term coolant is often used instead. When it comes to vehicles, antifreeze and coolant are the same thing – they are the same chemical(s); it just depends on the season or whether a person lives in a colder climate or warmer climate. While antifreeze/coolant will prevent water from freezing when the ambient temperature is very cold, it will also prevent the water in the radiator from boiling over when the ambient temperature is very warm. Should the water in a radiator and engine block boil away, it will cause the engine to overheat causing certain parts attached directly to the engine to warp or gaskets to blow out.
Osmotic Pressure Elevation
Before the final colligative property is introduced, a new concept needs to be presented. A semipermeable membrane is a thin membrane that allows certain small molecules to pass through, but not others. A thin sheet of cellophane, for example, acts as a semipermeable membrane (see Figure \(\PageIndex{3}\)(a)).
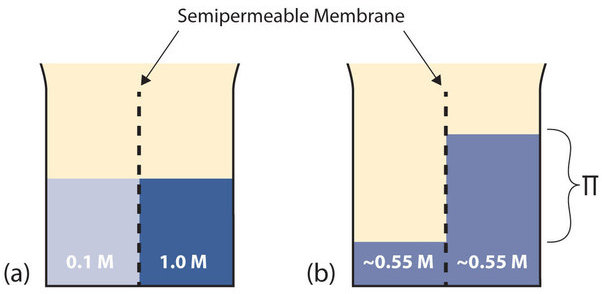
The semipermeable membrane is used to separate two solutions having the different concentrations marked. Curiously, this situation is not stable; there is a tendency for water molecules to move from the dilute side (on the left) to the concentrated side (on the right) until the concentrations are equalized, as in Figure \(\PageIndex{3}\)(b). This tendency is called osmosis. In osmosis, the solute is unable to pass through the membrane and remains on its original side of the system; only solvent molecules move through the semipermeable membrane. In the end, the two sides of the system will have different volumes. Because a column of liquid exerts a pressure, there is a pressure difference (Π) between the two sides of the system that is proportional to difference in height between the two columns. This pressure difference is called the osmotic pressure.
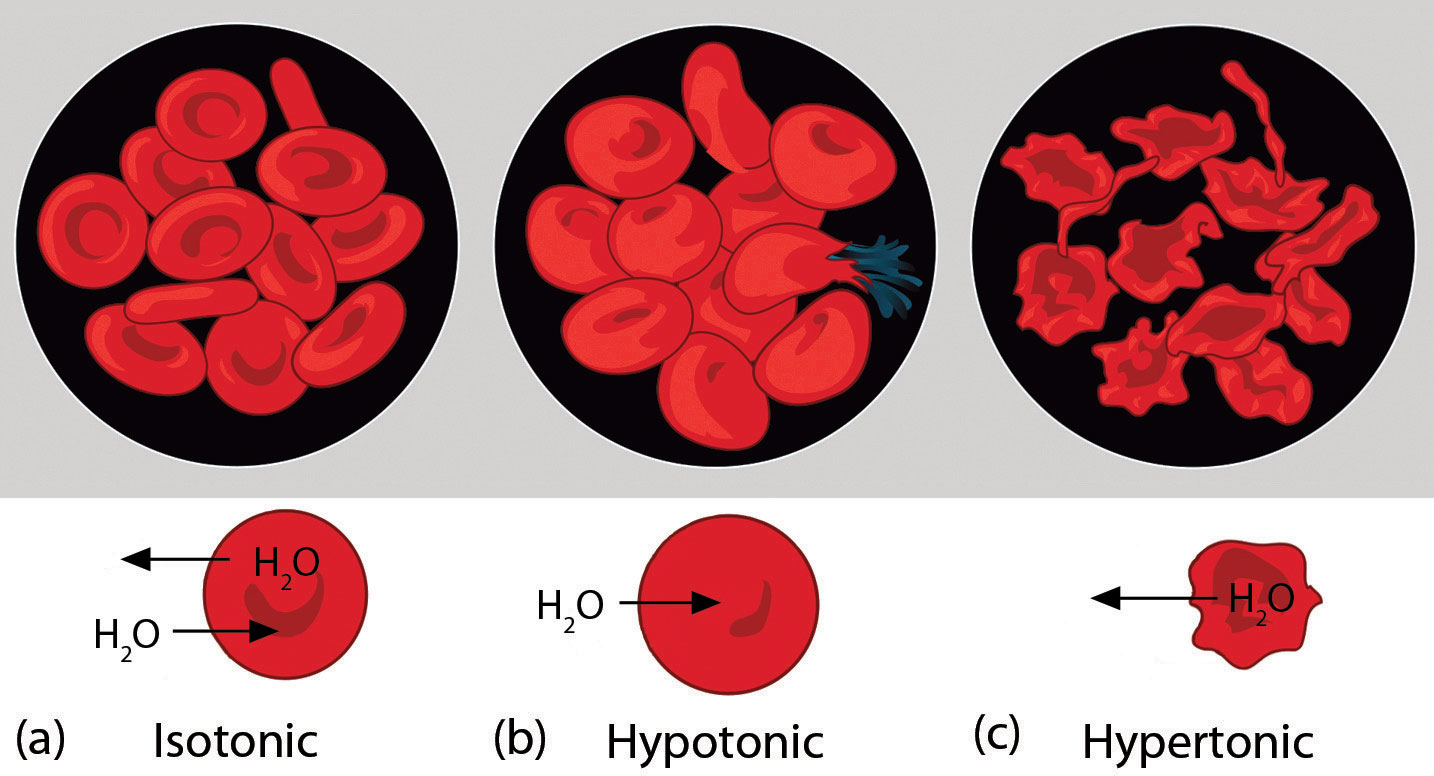
Osmotic pressure plays an important role in biological systems because cell walls are semipermeable membranes. In particular, when a person is receiving intravenous (IV) fluids, the osmotic pressure of the fluid needs to be approximately the same as blood serum to avoid any negative consequences. Figure \(\PageIndex{4}\) shows three red blood cells:
- A healthy red blood cell.
- A red blood cell that has been exposed to a lower solute concentration than normal blood serum (a hypotonic solution); the cell has plumped up as solvent moves into the cell to dilute the solutes inside.
- A red blood cell exposed to a higher solute concentration than normal blood serum (hypertonic); water leaves the red blood cell, so it collapses onto itself. Only when the solutions inside and outside the cell are the same (isotonic) will the red blood cell be able to do its job.
Osmotic pressure is also the reason you should not drink seawater if you’re stranded on a lifeboat in the ocean; seawater has a higher osmotic pressure than most of the fluids in your body. You can drink the water, but ingesting it will pull water out of your cells as osmosis works to dilute the seawater. Ironically, your cells will die of thirst, and you will also die. (It is okay to drink the water if you are stranded on a body of freshwater, at least from an osmotic pressure perspective.) Osmotic pressure is also thought to be important – in addition to capillary action – in getting water to the tops of tall trees.
Summary
- Colligative properties are properties that are due only to the number of particles in solution, and are not related to the chemical properties of the solute.
- Freezing points of solutions are lower than the freezing points of the pure solvents.
- Boiling points of solutions are higher than the boiling points of the pure solvents.
- Osmotic pressure is caused by concentration differences between solutions separated by a semipermeable membrane, and is an important biological consideration.
This page is shared under a CK-12 license and was authored, remixed, and/or curated by Melissa Alviar-Agnew, Henry Agnew, and Lance S. Lund (Anoka-Ramsey Community College). Original source: https://www.ck12.org/c/chemistry/.



