11.6: Writing Lewis Structures for Molecular Compounds
- Page ID
- 289427
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)⚙️ Learning Objectives
- Draw Lewis structures for molecular compounds.
By now, you have probably noticed a pattern in covalent bond formation. The normal number of covalent bonds for atoms in compounds is equal to the number of electrons required by an atom to complete its valence shell, as shown in Table \(\PageIndex{1}\). Recall that only nonmetals and/or metalloids typically form covalent bonds with each other.
When atoms form the normal number of covalent bonds with other elements, they may do so in any manner that sums to equal the normal number of bonds. For example, carbon (C) is located in Group IVA. This means it has four valence electrons and normally makes four covalent bonds. As a result, when carbon bonds with other elements, all of the following bond combinations are possible:
- 4 single bonds
- 2 single bonds and 1 double bond
- 1 single bond and 1 triple bond
- 2 double bonds
Further study in chemistry would reveal that the number of bonds an atom makes may differ when looking at polyatomic ions or in instances where the octet rule may be exceeded. Your instructor may choose to supplement this text with additional resources that examine polyatomic ions or expanded octets.
Here are some guidelines for constructing Lewis dot structures for more complex molecules than have been examined thus far:
☞ The Basics of Constructing Lewis Dot Structures
- Calculate the sum of the valence electrons in the molecule.
- Construct a skeleton structure for the molecule, connecting atoms with a dash/line. Remember that each dash/line counts as two electrons.
- The central atom(s) will usually be the element that makes the greatest number of bonds and is surrounded by atoms that make a lesser number of bonds.
- Bond angles around a central atom are rarely 180° (linear). The bond angle will only be linear when a central atom that is bonded to two other atoms has zero lone pairs (performed in the final check).
- Arrange the atoms in the skeleton structure so each atom makes the normal number of covalent bonds. If necessary, use double bonds or triple bonds to achieve the normal number of covalent bonds for each atom.
- Treat it like a puzzle that only fits together one way. In many cases, it is helpful to think of symmetry. A molecule with a few atoms is more likely to be build symmetrically around a central atom rather than in a straight line.
- Add electrons as pairs to make an octet around each atom in the molecule, starting with the surrounding atoms and working inward to the central atom(s).
- Remember that hydrogen follows the duet rule. Its valence shell holds up to two electrons.
- Remember that an atom has access to all of the electrons in the bonds made by that atom.
- Final check:
- The total number of electrons shown in the molecule must equal the number of valence electrons calculated in the first step.
- The octet rule is followed by every atom in the molecule (Note: hydrogen follows the duet rule).
- Every atom in the molecule makes the expected number of bonds.
- Change the bond angle to 180° (linear) for any central atom that has zero lone pairs and is also bonded to two other atoms.
✅ Example \(\PageIndex{1}\)
Write the Lewis dot structure for NF3.
Solution
| Steps for Writing Lewis Structures | |
|---|---|
|
1 N atom = 1 × 5 = 5 valence e– 3 F atoms = 3 × 7 = 21 valence e– sum of valence e– = 26 valence e– |
|
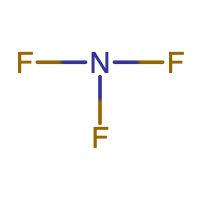 N is the central atom since it makes the most bonds. An N atom makes 3 bonds, while an F atom makes 1 bond. |
|
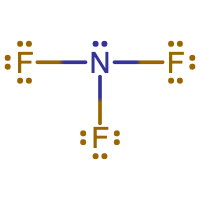 |
|
There are 26 electrons shown in the molecule. This matches the 26 valence electrons calculated in the first step. The N atom and all 3 F atoms are following the octet rule. N is making 3 bonds, as expected. Each F atom is making 1 bond, as expected. |
✅ Example \(\PageIndex{2}\)
Write the Lewis dot structure for COCl2.
Solution
| Steps for Writing Lewis Structures | |
|---|---|
|
1 C atom = 1 × 4 = 4 valence e– 1 O atom = 1 × 6 = 6 valence e– 2 Cl atoms = 2 × 7 = 14 valence e– sum of valence e– = 24 valence e– |
|
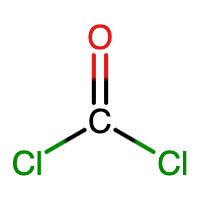 C is the central atom since it makes the most bonds (4 bonds). An O atom makes 2 bonds, while a Cl atom makes 1 bond. |
|
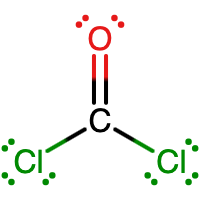 |
|
There are 24 electrons shown in the molecule. This matches the 24 valence electrons calculated in the first step. The C atom, O, and both Cl atoms are following the octet rule. C is making 4 bonds and O is making 2 bonds, as expected. Each Cl atom is making 1 bond, as expected. |
✅ Example \(\PageIndex{3}\)
Write the Lewis dot structure for HCN.
Solution
| Steps for Writing Lewis Structures | |
|---|---|
|
1 H atom = 1 × 1 = 1 valence e– 1 C atom = 1 × 4 = 4 valence e– 1 N atom = 1 × 5 = 5 valence e– sum of valence e– = 10 valence e– |
|
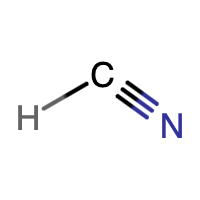 C is the central atom since it makes the most bonds (4 bonds). An N atom makes 3 bonds, while an H atom makes 1 bond. |
|
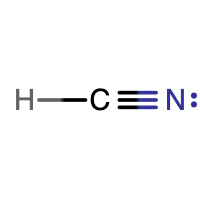 |
|
There are 10 electrons shown in the molecule. This matches the 10 valence electrons calculated in the first step. H is following the duet rule. Both C and N are following the octet rule. H is making 1 bond, C is making 4 bonds, and N is making 3 bonds. These are all the expected number of bonds. Also, the molecule is linear, since the central C atom is only bonded to two other atoms and there are no lone pairs on the C. |
✏️ Exercise \(\PageIndex{1}\)
Write Lewis dot structures for each.
- SI2
- CO2
- CH2O
- C2H3F3
- Answer A
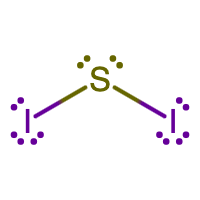
- Answer B
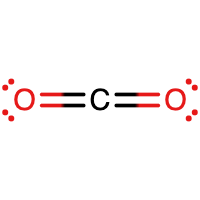
- Answer C
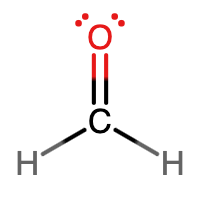
- Answer D
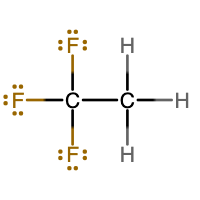
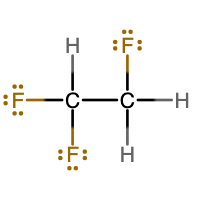
Either of these two Lewis structures are acceptable. They are isomers of each other. Isomers are substances that have the same chemical formula, but a different molecular structure.
Notice that the structure on the left has 3 F atoms attached to one C atom and 3 H atoms attached to the other C atom. The structure on the right has 2 F atoms and 1 H atom attached to one carbon atom and 1 F atom and 2 H atoms attached to the other C atom.
This page is shared under a CK-12 license and was authored, remixed, and/or curated by Lance S. Lund (Anoka-Ramsey Community College), Josh Halpern, Melissa Alviar-Agnew, and Henry Agnew. Original source: https://www.ck12.org/c/chemistry/.



