7.9: Acid-Base and Gas Evolution Reactions
- Page ID
- 289400
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)⚙️ Learning Objectives
- Identify when a reaction will evolve a gas.
Neutralization Reactions
Acids and bases react chemically with each other to form salts. A salt is a general chemical term for any ionic compound formed from an acid and a base. In reactions where the acid is a hydrogen-ion-containing compound and the base is a hydroxide-ion-containing compound, water is also a product. The general reaction is as follows:
acid + base → salt + water
The reaction of acid and base to make water and a salt is called neutralization. Like any chemical equation, a neutralization chemical equation must be properly balanced. For example, the neutralization reaction between sodium hydroxide and hydrochloric acid is as follows:
NaOH (aq) + HCl (aq) → NaCl (aq) + H2O (l)
with coefficients all understood to be one. The neutralization reaction between sodium hydroxide and sulfuric acid is as follows:
2 NaOH (aq) + H2SO4 (aq) → Na2SO4 (aq) + 2 H2O (l)
✅ Example \(\PageIndex{1}\): Neutralizing Nitric Acid
Nitric acid, HNO3 (aq), can be neutralized by calcium hydroxide, Ca(OH)2 (aq). Write a balanced chemical equation for the reaction between these two compounds and identify the salt that it produces.
Solution
Write the unbalanced equation. This is a double replacement reaction, so the cations and anions swap to create new products. Referring to the solubility rules, Ca(NO3)2 is soluble in water resulting in a phase label of (aq). Water, H2O, is a liquid at room temperature resulting in a phase label of (l).
Ca(OH)2 (aq) + HNO3 (aq) → Ca(NO3)2 (aq) + H2O (l)
At this point, the equation may be balanced by placing a coefficient of "2" in front of HNO3 (aq) and H2O (l).
Ca(OH)2 (aq) + 2 HNO3 (aq) → Ca(NO3)2 (aq) + 2 H2O (l)
The salt formed is calcium nitrate, Ca(NO3)2 (aq).
✏️ Exercise \(\PageIndex{1}\)
Hydrobromic acid, HBr (aq), may be neutralized with potassium hydroxide, KOH (aq). Write a balanced chemical equation for the reaction between these two compounds and identify the salt that it produces.
- Answer
- KOH (aq) + HBr (aq) → KBr (aq) + H2O (l)
The salt is potassium bromide, KBr (aq).
Gas Evolving Reactions
Figure \(\PageIndex{1}\) shows an apparatus that may be used for collecting gases in the laboratory.
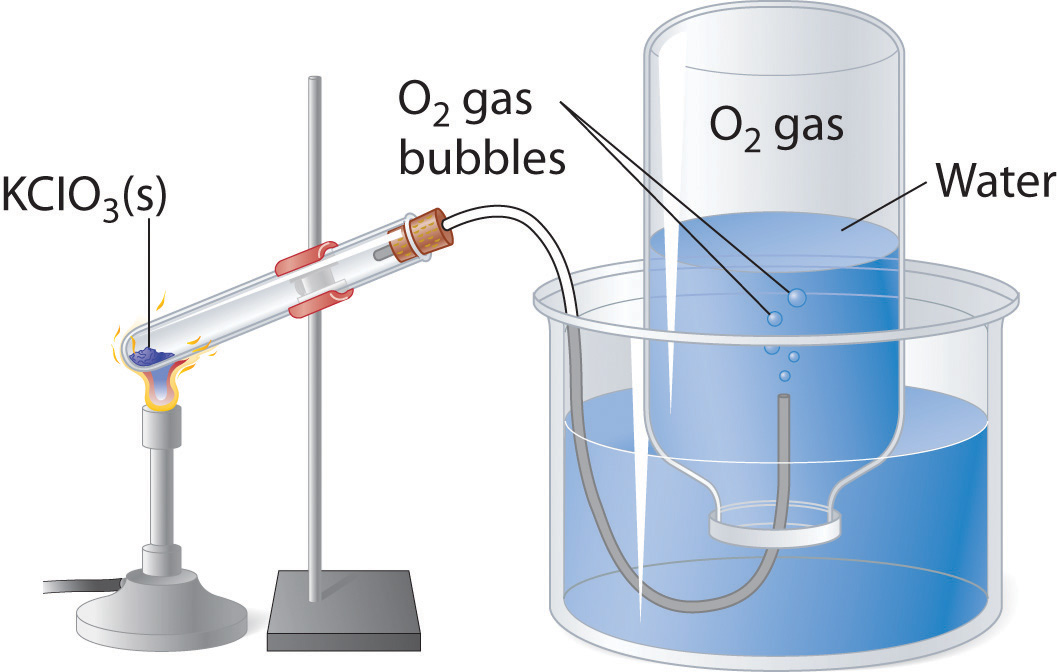
A gas evolution reaction is a chemical process that produces a gas, such as hydrogen or carbon dioxide. In the following examples, an acid reacts with a carbonate, producing salt, carbon dioxide, and water, respectively. For example, nitric acid reacts with sodium carbonate to form sodium nitrate, carbon dioxide, and water (Table \(\PageIndex{1}\)):
2 HNO3 (aq) + Na2CO3 (aq) → 2 NaNO3 (aq) + CO2 (g) + H2O (l)
Hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water:
2 HCl (aq) + CaCO3 (aq) → CaCl2 (aq) + CO2 (g) + H2O (l)
The oxidation of metals by strong acids is another common example of a gas evolution reaction. This reaction will yield a metal salt and hydrogen gas.
2 HCl (aq) + Zn (s) → ZnCl2 (aq) + H2 (g)
Here, hydrochloric acid oxidizes zinc to produce an aqueous metal salt and hydrogen gas bubbles.
This page is shared under a CC BY 4.0 and CK-12 licenses and was authored, remixed, and/or curated by Boundless (www.boundless.com), Wikipedia, Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors, Marisa Alviar-Agnew & Henry Agnew (OpenStax) and Lance S. Lund (Anoka-Ramsey Community College) via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request.



