7.8: Precipitation Reactions
- Page ID
- 289398
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)⚙️ Learning Objectives
- To identify a precipitation reaction and predict solubility.
A precipitation reaction is a reaction that yields an insoluble product – a precipitate – when two solutions are mixed. When a colorless solution of silver nitrate is mixed with a yellow-orange solution of potassium dichromate, a reddish precipitate of silver dichromate is produced (see Video \(\PageIndex{1}\)).
2 AgNO3 (aq) + K2Cr2O7 (aq) → Ag2Cr2O7 (s) + 2 KNO3 (aq)
This chemical equation has the general form of an exchange reaction:
\[\ce{ AC + BD \rightarrow }\underset{insoluble}{\ce{AD}} + \ce{BC}\]
Thus precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Because both components of each compound change partners, such reactions are sometimes called double-replacement reactions. Precipitation reactions are used to isolate metals that have been extracted from their ores, and to recover precious metals for recycling.
Just as important as predicting the product of a reaction is knowing when a chemical reaction will not occur. Simply mixing solutions of two different chemical substances does not guarantee that a reaction will take place. For example, if 500 mL of an aqueous sodium chloride solution is mixed with 500 mL of an aqueous potassium bromide solution, the final solution has a volume of 1000 mL and contains a mixture of Na+ (aq), Cl− (aq), K+ (aq), and Br− (aq) ions. As you will see in (Figure \(\PageIndex{1}\)), none of these species reacts with any of the others. When these solutions are mixed, the only effect is to dilute each solution with the other.
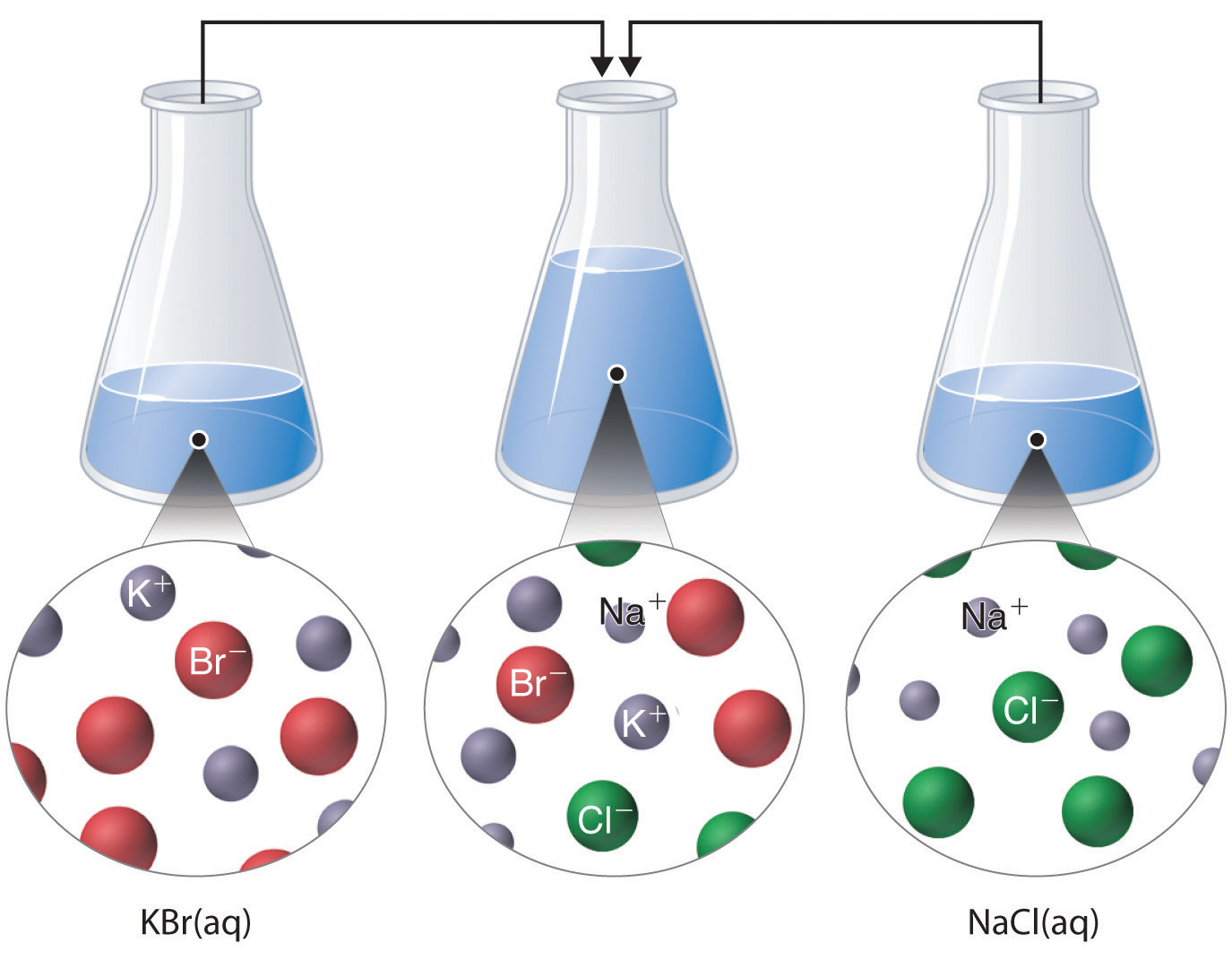
Predicting Precipitation Reactions
A precipitation reaction occurs when a solid precipitate forms after mixing two strong electrolyte solutions. As stated previously, if none of the species in the solution reacts then no net reaction occurs.
When two aqueous ionic compounds are mixed together, there will be no reaction when both of the potential products would also be aqueous ionic compounds.
Let's see what happens when aqueous solutions of barium chloride and lithium sulfate are mixed:
BaCl2 (aq) + Li2SO4 (aq) →
Since barium chloride and lithium sulfate are both strong electrolytes, each dissociates completely in water resulting in a solution that contains all of the constituent anions and cations. Barium chloride is comprised of barium ions, Ba2+, and chloride ions, Cl–, while lithium sulfate is comprised of lithium ions, Li+, and sulfate ions, SO42−. The anions exchange partners.

Barium ions pair up with sulfate ions forming barium sulfate, BaSO4 (Ba2+ balances the charge of SO42−). Lithium ions pair up with chloride ions forming LiCl (Li+ balances the charge of Cl–).
BaCl2 (aq) + Li2SO4 (aq) → BaSO4 + LiCl
Referring to the solubility rules found in the previous section, BaSO4 is insoluble in water resulting in a phase label of (s), while LiCl is soluble in water resulting in a phase label of (aq).
BaCl2 (aq) + Li2SO4 (aq) → BaSO4 (s) + LiCl (aq)
At this point, the equation may be balanced by placing a coefficient of "2" in front of LiCl (aq).
BaCl2 (aq) + Li2SO4 (aq) → BaSO4 (s) + 2 LiCl (aq)
Although soluble barium salts are toxic, BaSO4 is so insoluble that it can be used to diagnose stomach and intestinal problems without being absorbed into tissues. An outline of the digestive organs appears on x-rays of patients who have been given a “barium milkshake” or a “barium enema”, a suspension of very fine BaSO4 particles in water.

Figure \(\PageIndex{2}\): An x-ray of the digestive organs of a patient who has swallowed a “barium milkshake.” A barium milkshake is a suspension of very fine BaSO4 particles in water; the high atomic mass of barium makes it opaque to x-rays. (OpenStax, CC BY 4.0, via Wikimedia Commons)
Complete and balance the following equation. If no reaction takes place, write "No Reaction" after the arrow.
MgBr2 (aq) + (NH4)2CO3 (aq) →
Solution
MgBr2 is called magnesium bromide and is comprised of magnesium ions, Mg2+, and bromide ions, Br−, while (NH4)2CO3 is called ammonium carbonate and is comprised of ammonium ions, NH4+, and carbonate ions, CO32–. The anions exchange partners.

Magnesium ions pair up with carbonate ions forming magnesium carbonate, MgCO3 (Mg2+ balances the charge of CO32–). Ammonium ions pair up with bromide ions forming NH4Br (NH4+ balances the charge of Br–).
MgBr2 (aq) + (NH4)2CO3 (aq) → MgCO3 + NH4Br
Referring to the solubility rules, MgCO3 is insoluble in water resulting in a phase label of (s), while NH4Br is soluble in water resulting in a phase label of (aq).
MgBr2 (aq) + (NH4)2CO3 (aq) → MgCO3 (s) + NH4Br (aq)
At this point, the equation may be balanced by placing a coefficient of "2" in front of NH4Br (aq).
MgBr2 (aq) + (NH4)2CO3 (aq) → MgCO3 (s) + 2 NH4Br (aq)
Write a balanced chemical equation that shows what happens when aqueous solutions of sodium carbonate and potassium iodide are mixed. If there is no reaction, write "No Reaction" after the arrow.
Solution
Sodium carbonate is comprised of sodium ions, Na+, and carbonate ions, CO32–, while potassium iodide is comprised of potassium ions, K+, and iodide ions, I−. The anions exchange partners.

Sodium ions pair up with iodide ions forming sodium iodide, NaI (Na+ balances the charge of I−). Potassium ions pair up with carbonate ions forming K2CO3 (two K+ balances the charge of one CO32–).
Na2CO3 (aq) + KI (aq) → NaI + K2CO3
Referring to the solubility rules, both NaI and K2CO3 are soluble in water resulting in a phase label of (aq).
Na2CO3 (aq) + KI (aq) → NaI (aq) + K2CO3 (aq)
Since this equation shows two aqueous ionic compounds being mixed together to potentially yield two aqueous ionic compounds, no reaction will take place.
Na2CO3 (aq) + KI (aq) → No Reaction
Write a balanced chemical equation that shows what happens when aqueous solutions of iron(III) nitrate and sodium hydroxide are mixed. If there is no reaction, write "No Reaction" after the arrow.
Solution
Iron(III) nitrate is comprised of iron(III) ions, Fe3+, and nitrate ions, NO3–, while sodium hydroxide is comprised of sodium ions, Na+, and hydroxide ions, OH−. The anions exchange partners.
_nitrate_and_sodium_hydroxide.png?revision=1&size=bestfit&width=350&height=34)
Iron(III) ions pair up with hydroxide ions forming iron(III) hydroxide, Fe(OH)3 (one Fe3+ balances the charge of three OH−). Sodium ions pair up with nitrate ions forming NaNO3 (Na+ balances the charge of NO3–).
Fe(NO3)3 (aq) + NaOH (aq) → Fe(OH)3 + NaNO3
Referring to the solubility rules, Fe(OH)3 is insoluble in water resulting in a phase label of (s), while NaNO3 is soluble in water resulting in a phase label of (aq).
Fe(NO3)3 (aq) + NaOH (aq) → Fe(OH)3 (s) + NaNO3 (aq)
At this point, the equation may be balanced by placing a coefficient of "3" in front of NaOH (aq) and NaNO3 (aq).
Fe(NO3)3 (aq) + 3 NaOH (aq) → Fe(OH)3 (s) + 3 NaNO3 (aq)
Complete and balance each equation. If no reaction takes place, write "No Reaction" after the arrow.
- Li3PO4 (aq) + KOH (aq) →
- MgSO4 (aq) + SrI2 (aq) →
- Answer A
- Li3PO4 (aq) + KOH (aq) → No Reaction
- Answer B
- MgSO4 (aq) + SrI2 (aq) → SrSO4 (s) + MgI2 (aq)
Write a balanced chemical equation that shows what happens when aqueous solutions of each pair are mixed. If no reaction occurs, write "No Reaction" after the arrow.
- Potassium phosphate and calcium chloride
- Zinc nitrate and sodium carbonate
- Answer A
- 2 K3PO4 (aq) + 3 CaCl2 (aq) → Ca3(PO4)2 (s) + 6 KCl (aq)
- Answer B
- Zn(NO3)2 (aq) + Na2CO3 (aq) → ZnCO3 (s) + 2 NaNO3 (aq)
Summary
- In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when two electrolyte solutions are mixed.
- To predict the product of a precipitation reaction, all species initially present in the solutions are identified, as are any combinations likely to produce an insoluble salt.
This page is shared under a CK-12 license and was authored, remixed, and/or curated by Joshua Halpern (Howard University), Melissa Alviar-Agnew, Henry Agnew, and Lance S. Lund (Anoka-Ramsey Community College). Original source: https://www.ck12.org/c/chemistry/.



