5.5: Binary Ionic Compounds
- Page ID
- 289375
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)⚙️ Learning Objectives
- Write the correct names and formulas for binary ionic compounds.
Ionic compounds do not exist as molecules. In the solid state, ionic compounds are in a crystal lattice containing many ions each of the cation and anion. An ionic formula, like NaCl, is an empirical formula. This formula merely indicates that sodium chloride is made of an equal number of sodium and chloride ions. Sodium sulfide, another ionic compound, has the formula Na2S. This formula indicates that this compound is made up of twice as many sodium ions as sulfide ions. This section will teach you how to write the names of binary ionic compounds, as well as to determine the correct ratio of ions, so that you may correctly write a formula.
Writing Chemical Names
As we recall, a binary ionic compound is one that contains two elements, one of which is a metal and the other of which is a nonmetal. The metal is always written first in the name and formula followed by the nonmetal. The convention for naming binary ionic compounds is
- Metals retain their name.
- Nonmetals retain their root name followed by a suffix of -ide.
The root name represents the first one or two syllables of an element's name:
| Symbol | Name | Root Name | Symbol | Name | Root Name |
|---|---|---|---|---|---|
| H | hydrogen | hydr- | P | phosphorus | phosph- |
| C | carbon | carb- | S | sulfur | sulf- |
| N | nitrogen | nitr- | Cl | chlorine | chlor- |
| O | oxygen | ox- | Br | bromine | brom- |
| F | fluorine | fluor- | I | iodine | iod- |
✅ Example \(\PageIndex{1}\): Writing Chemical Names
Write the name for each compound.
- Al2S3
- BaBr2
- K3N
Solution
- Al = aluminum. S = sulfur. The root name of sulfur is sulf-. The nonmetal has a suffix of -ide. Al2S3 is aluminum sulfide.
- Ba = barium. Br = bromine. The root name of bromine is brom-. The nonmetal has a suffix of -ide. BaBr2 is barium bromide.
- K = potassium. N = nitrogen. The root name of nitrogen is nitr-. The nonmetal has a suffix of -ide. K3N is potassium nitride.
Writing Chemical Formulas
If you know the name of a binary ionic compound, you can write its chemical formula. Start by writing the metal ion with its charge, followed by the nonmetal ion with its charge. Because the overall compound must be electrically neutral, decide how many of each ion is needed in order for the positive and negative charges to balance each other. If the charges of the ions are 2+ and 2–, nothing needs to be done – the charges are already balanced. If the charges of the ions are 3+ and 1–, three of the ions with the 1– charge are needed to balance one of the ions with the 3+ charge.
When balancing charges, many find it easiest to think of the least common multiple between the magnitude of charge. For example, if the charges of the ions are 2+ and 3–, the least common multiple between 2 and 3 is 6. Therefore, the charge will be balanced when the positive charges sum to 6 and the negative charges sum to 6.
The ions shown in Figure \(\PageIndex{1}\) have fixed charges related to their position on the periodic table, as previously discussed in Section 4.8. Note that most main-group elements (groups labeled with Roman numerals followed by the letter A) have fixed charges, while most transition metals (groups labeled with Roman numerals followed by the letter B) have charges that are variable. Metals that have variable charges are discussed in the next section.
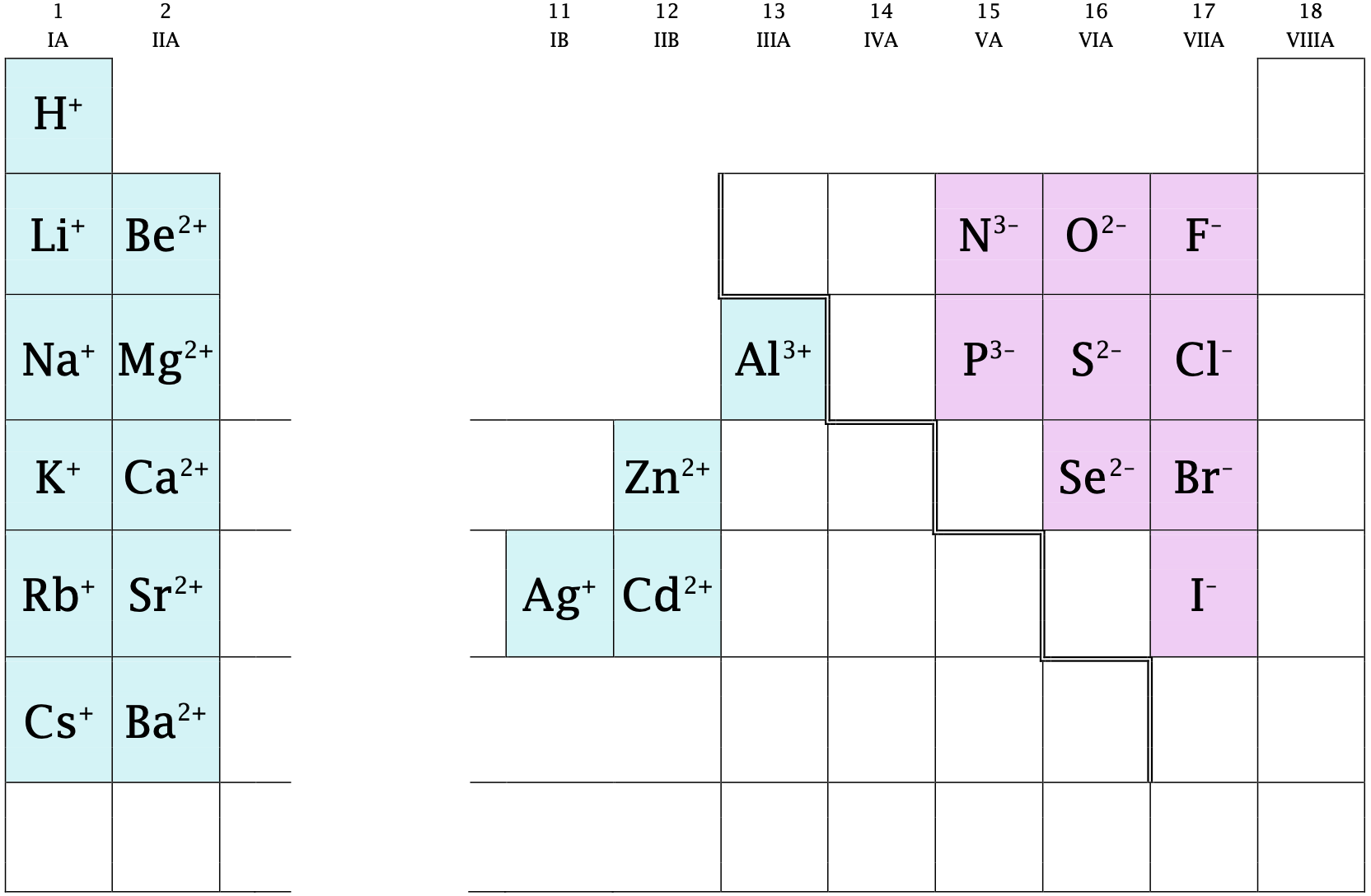
Figure \(\PageIndex{1}\): Ions with fixed charges related to position on the periodic table.
✅ Example \(\PageIndex{2}\): Writing Chemical Formulas
Write the chemical formulas for:
- lithium fluoride
- sodium oxide
- strontium iodide
Solution
| lithium fluoride | sodium oxide | strontium iodide | |||
|---|---|---|---|---|---|
|
Li+ F– | Na+ O2– | Sr2+ I– | ||
|
Li+ F– | Na+ Na+ |
O2– | Sr2+ | I– I– |
|
1+ balances 1– | 2+ balances 2– | 2+ balances 2– | ||
|
LiF | Na2O | SrI2 | ||
✅ Example \(\PageIndex{3}\): Writing Chemical Formulas
Write the chemical formulas for:
- magnesium sulfide
- aluminum oxide
Solution
| magnesium sulfide | aluminum oxide | ||
|---|---|---|---|
|
Mg2+ S2– | Al3+ O2– | |
|
Mg2+ S2– | Al3+ Al3+ |
O2– O2– O2– |
|
2+ balances 2– | 6+ balances 6– | |
|
MgS | Al2O3 | |
✏️ Exercise \(\PageIndex{1}\)
If the chemical formula is provided, write the name. If the name is provided, write the formula.
- Li2S
- Ca3N2
- potassium phosphide
- barium oxide
- Answer A
- lithium sulfide
- Answer B
- calcium nitride
- Answer C
- K3P
- Answer D
- BaO
Summary
- Formulas for ionic compounds contain the symbols and number of each atom present in a compound in the lowest whole number ratio.
- Ionic compounds must be neutral overall.
This page is shared under a CK-12 license and was authored, remixed, and/or curated by Lance S. Lund (Anoka-Ramsey Community College), Melissa Alviar-Agnew, and Henry Agnew. Original source: https://www.ck12.org/c/chemistry/.



