4.9: Ion Formation
- Page ID
- 289367
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)⚙️ Learning Objectives
- Define the two types of ions.
Up until now, we have only considered atoms that are neutral, where the number of electrons is equal to the number of protons. As we recall, an atom consists of a positively charged nucleus that contains the protons and neutrons that is surrounded by a cloud of negatively charged electrons (Figure \(\PageIndex{1}\)). This makes the electrons more accessible, resulting in their ability to more readily be gained or lost. This is also why it is the number of protons in the nucleus that determines the identity of the element – the number of protons does not change.

Figure \(\PageIndex{1}\): Helium atom showing the nucleus surrounded by an electron cloud. Cepheiden, CC BY-SA 3.0, via Wikimedia Commons
As we will learn in Chapter 11, atoms tend to form chemical bonds by having complete outer shells of electrons. In some cases, it is easier for an atom to gain electrons to make an outer shell complete. In other cases, it is easier for an atom to lose outer shell electrons to arrive at a complete shell that lies below.
An atom that has gained or lost one or more electrons is called an ion. An ion always has a charge that is positive (+) or negative (−). An ion with a positive charge is called a cation, while an ion with a negative charge is called an anion. An easy mnemonic to distinguish the two is that the the word cation contains a plus sign (in other words, think of it as ca+ion).
Cations
Metals generally have three or fewer outer shell electrons making them more likely to lose electrons. Atoms that lose electrons acquire a positive charge since there are fewer of the negatively charged electrons that remain in the electron cloud compared to the positively charged protons that are present in the nucleus. Consider a sodium atom, which ordinarily loses one electron (Figure \(\PageIndex{2}\)). The cation produced in this manner, Na+, is called a sodium ion to distinguish it from a neutral atom of sodium.
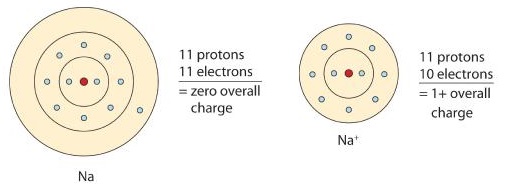
Also notice that the ion charge is written as 1+ rather than +1. The convention for showing charges on particles is to always write the sign after the number. Using this convention gives us the "heads up" that we are looking at the charge of a particle rather than an ordinary positive or negative number. If we want to summarize the process of sodium losing an electron to form the ion, we may write it like this:
\[\ce{Na \rightarrow Na^{+} + e^{-}}\]
In table salt, \(\mathrm{NaCl}\), this electron is "donated" to an atom of chlorine.
Anions
Nonmetals generally have four or more outer shell electrons making them more likely to gain electrons. Atoms that gain electrons acquire a negative charge since there would be more of the negatively charged electrons present in the electron cloud compared to the positively charged protons in the nucleus. Consider a chlorine atom, which ordinarily gains one electron (Figure \(\PageIndex{3}\)). The resulting anion, Cl–, is called the chloride ion. Note the slight change in the suffix (-ide instead of -ine) to create the name of this anion.
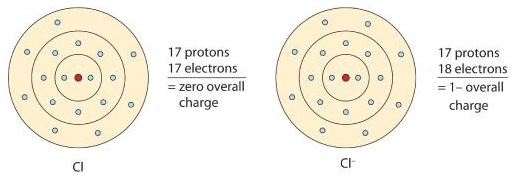
If we want to summarize the process of chlorine gaining an electron to form the ion, we may write it like this:
\[\mathrm{Cl}+\mathrm e^–\rightarrow\mathrm{Cl}^–\]
In table salt, this electron comes from the sodium atom.
Location, Location, Location!
In many cases, elements that belong to the same group on the periodic table form ions with the same charge because they have the same number of outer shell electrons. Thus, the periodic table becomes a tool for remembering the charges on many ions. For example, all ions made from alkali metals, the first column on the periodic table, have a 1+ charge. Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. On the other side of the periodic table, the next-to-last column, the halogens, form ions having a 1− charge. Figure \(\PageIndex{4}\) shows how the charge on many ions may be predicted its location.
Once again, note the convention of writing the sign after the number. The barium cation is written Ba2+, not Ba+2.
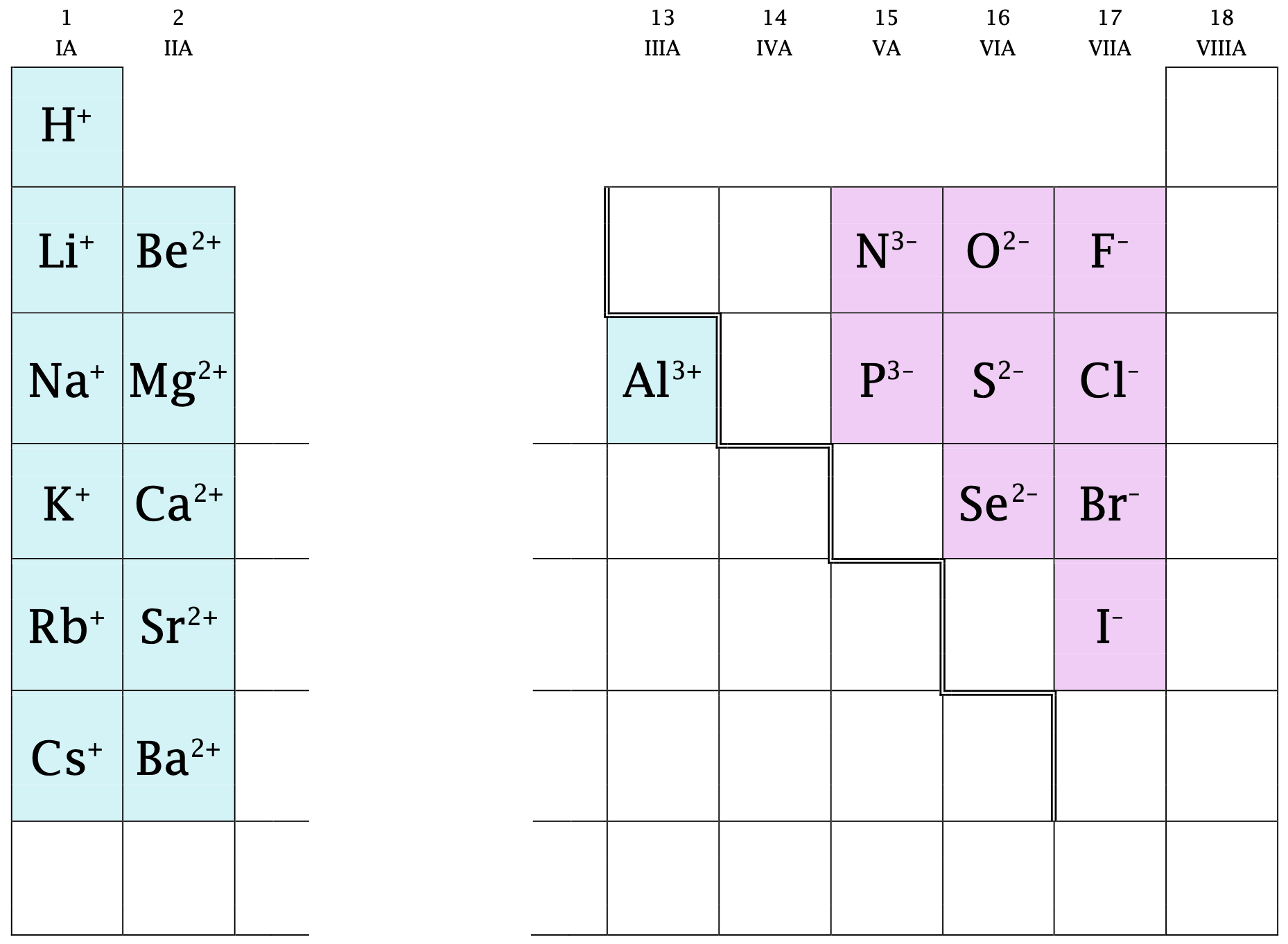
Figure \(\PageIndex{4}\): Predicting ionic charges. The charge an atom acquires when it becomes an ion is often related to its position on the periodic table.
✅ Example \(\PageIndex{1}\)
- How many protons and electrons are in a potassium ion, K+?
- What is the chemical symbol and charge of an ion that has 25 p+ and 23 e−?
Solution
- Potassium has an atomic number is 19, so there are 19 p+.
Since there is a single positive (+) charge on the ion, there must be one more positively charged proton in the nucleus than there are negatively charged electrons in the electron cloud.
Therefore, there are 18 e−.
- With 25 p+, the atomic number (Z) is 25. This identifies the element as manganese, Mn.
Since there are two more protons, p+, than electrons, e−, there is an excess of two positive (+) charges on the ion, meaning its charge is 2+.
Therefore, we write the symbol and charge as Mn2+.
✏️ Exercise \(\PageIndex{1}\)
How many protons, electrons, and neutrons are in each ion?
- How many protons and electrons are in a phosphide ion, P3–?
- What is the formula of an ion (its chemical symbol and charge) that has 53 p+ and 54 e−?
- Answer A
- 15 p+, 18 e−
- Answer B
- I–
This page is shared under a CK-12 license and was authored, remixed, and/or curated by Lance S. Lund (Anoka-Ramsey Community College), Melissa Alviar-Agnew, and Henry Agnew. Original source: https://www.ck12.org/c/chemistry/.



