4.11: Exercises
- Page ID
- 357319
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)4.1: Cutting Aluminum Until You Get Atoms
- Give the proper formula for each diatomic element.
- Answer
-
H2, N2, O2, F2, Cl2, Br2, I2
4.2: Early Atomic Theory
- In the following drawing, the green spheres represent atoms of a certain element. The purple spheres represent atoms of another element. If the spheres of different elements touch, they are part of a single unit of a compound. The following chemical change represented by these spheres may violate one of the ideas of Dalton’s atomic theory. Which one?
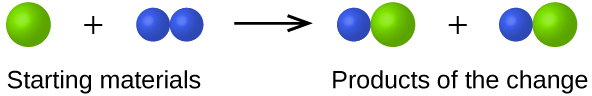
- Answer
-
The starting materials consist of one green sphere and two purple spheres. The products consist of two green spheres and two purple spheres. This violates Dalton’s postulate that that atoms are not created during a chemical change, but are merely redistributed.
4.3: Discovery of the Nucleus
- Explain how atoms are composed.
- Answer
-
Atoms have a small nucleus containing protons and neutrons. The nucleus is surrounded by "empty" space in which the electrons travel.
- Where is most of the mass of an atom located?
- Answer
-
in the nucleus
4.4: Protons, Neutrons, and Electrons
- Define atomic mass unit. What is its abbreviation?
- Answer
-
An atomic mass unit is \(\frac1{12}\) of the mass of a carbon-12 atom. Its abbreviation is amu.
- Which is larger, a proton or an electron?
- Answer
-
proton
- Which is larger, a neutron or an electron?
- Answer
-
neutron
- What are the charges for each of the three subatomic particles?
- Answer
-
proton = 1+
neutron = 0,
electron = 1–
- How are electrons and protons similar? How are they different?
- Answer
-
They are similar in that they are both charged particles. They are different in that the charges are different and they have different masses.
- How are protons and neutrons similar? How are they different?
- Answer
-
They are similar in that they both have a mass of approximately 1 amu. They are different in that protons have a 1+ charge and neutrons are neutral.
4.5: Chemical Symbols and the Atomic Number
- Define atomic number.
- Answer
-
The number of protons in the nucleus of one atom of an element.
- What is the atomic number for a boron atom?
- Answer
-
5
- What is the atomic number of helium?
- Answer
-
2
- Give the chemical symbol for each element.
- sodium
- argon
- nitrogen
- radon
- Answer
-
- Na
- Ar
- N
- Rn
- Give the chemical symbol for each element.
- silver
- gold
- mercury
- iodine
- Answer
-
- Ag
- Au
- Hg
- I
- Give the name of the element.
- Si
- Mn
- Fe
- Cr
- Answer
-
- silicon
- manganese
- iron
- chromium
- Give the name of the element.
- F
- Cl
- Br
- I
- Answer
-
- fluorine
- chlorine
- bromine
- iodine
4.6: The Periodic Table
- Distinguish between a metal and a nonmetal.
- Answer
-
Metals are typically shiny, conduct electricity and heat well, and are malleable and ductile; nonmetals are a variety of colors and phases, are brittle in the solid phase, and do not conduct heat or electricity well.
- What properties do metalloids have?
- Answer
-
Metalloids have properties that are intermediate between metals and nonmetals.
- Why is iron considered a metal?
- Answer
-
Iron is a metal because it is solid, is shiny, and conducts electricity and heat well.
- Why is oxygen considered a nonmetal?
- Answer
-
Oxygen is a gas. It is not a good conductor of heat or electricity.
- Elemental carbon is a black, dull-looking solid that conducts heat and electricity well. It is very brittle and cannot be made into thin sheets or long wires. Of these properties, how does carbon behave as a metal? How does carbon behave as a nonmetal?
- Answer
-
Carbon behaves as a metal because it conducts heat and electricity well. It is a nonmetal because it is black and brittle and cannot be made into sheets or wires.
- Pure silicon is shiny and silvery but does not conduct electricity or heat well. Of these properties, how does silicon behave as a metal? How does silicon behave as a nonmetal?
- Answer
-
Silicon behaves as a metal because it is silver and shiny. It behaves as a nonmetal because it does not conduct heat or electricity well.
- Use its place on the periodic table to determine if indium, In, atomic number 49, is a metal or a nonmetal.
- Answer
-
metal
- Only a few atoms of astatine, At, atomic number 85, have been detected. On the basis of its position on the periodic table, would you expect it to be a metal or a nonmetal?
- Answer
-
It is a metalloid. However, between the two options, it would behave more like a nonmetal than a metal.
- Using the periodic table, classify each of the following elements as a metal or a nonmetal, and then further classify each as a main-group (representative) element, transition metal, or inner transition metal:
- uranium
- bromine
- strontium
- neon
- gold
- americium
- rhodium
- sulfur
- carbon
- potassium
- Answer
-
- metal, inner transition metal
- nonmetal, main-group element
- metal, main-group element
- nonmetal, main-group element
- metal, transition metal
- metal, inner transition metal
- metal, transition metal
- nonmetal, main-group element
- nonmetal, main-group element
- metal, main-group element
- Using the periodic table, classify each of the following elements as a metal or a nonmetal, and then further classify each as a main-group (representative) element, transition metal, or inner transition metal:
- cobalt
- europium
- iodine
- indium
- lithium
- oxygen
- cadmium
- terbium
- rhenium
- Answer
-
- metal, transition metal
- metal, inner transition metal
- nonmetal, main-group element
- metal, main-group element
- metal, main-group element
- nonmetal, main-group element
- metal, transition metal
- metal, inner transition metal
- metal, transition metal
- Using the periodic table, identify the lightest member of each of the following groups:
- noble gases
- alkaline earth metals
- alkali metals
- Answer
-
- He
- Be
- Li (as H is not technically an alkali metal)
- Using the periodic table, identify the heaviest member of each of the following groups:
- alkali metals
- noble gases
- alkaline earth metals
- Answer
-
- Fr
- Og
- Ra
- Use the periodic table to give the name and symbol for each of the following elements:
- the noble gas in the same period as germanium
- the alkaline earth metal in the same period as selenium
- the halogen in the same period as lithium
- Answer
-
- Kr, krypton
- Ca, calcium
- F, fluorine
- Use the periodic table to give the name and symbol for each of the following elements:
- the halogen in the same period as the alkali metal with 11 protons
- the alkaline earth metal in the same period with the neutral noble gas with 18 electrons
- the noble gas in the same row as an isotope with 25 protons
- the noble gas in the same period as gold
- Answer
-
- Cl, chlorine
- Mg, magnesium
- Kr, krypton
- Rn, radon
- Write a symbol for each of the following neutral isotopes. Include the atomic number and mass number for each.
- the alkali metal with 11 protons and a mass number of 23
- the noble gas element with 75 neutrons in its nucleus and 54 electrons in the neutral atom
- the isotope with 33 protons and 40 neutrons in its nucleus
- the alkaline earth metal with 88 electrons and 138 neutrons
- the noble gas, used in lighting, with 10 electrons and 10 neutrons
- the lightest alkali metal with three neutrons
- Answer
-
- \(\ce{^{23}_11Na}\)
- \(\ce{^{129}_54Xe}\)
- \(\ce{^{73}_33As}\)
- \(\ce{^{226}_88Ra}\)
- \(\ce{^{20}_10Ne}\)
- \(\ce{^{6}_3Li}\)
4.7: Isotopes and Mass Numbers
- Which pair represents isotopes?
- \(\ce{^{4}_2He}\) and \(\ce{^{3}_2He}\)
- \(\ce{^{56}_26Fe}\) and \(\ce{^{56}_25Mn}\)
- \(\ce{^{28}_14Si}\) and \(\ce{^{31}_15P}\)
- Answer
-
pair a
- Which pair represents isotopes?
- \(\ce{^{40}_20Ca}\) and \(\ce{^{40}_19K}\)
- \(\ce{^{56}_26Fe}\) and \(\ce{^{56}_28Fe}\)
- \(\ce{^{238}_92U}\) and \(\ce{^{235}_92U}\)
- Answer
-
pair c
- Give complete symbols of each atom, including the atomic number and the mass number.
- an oxygen atom with 8 protons and 8 neutrons
- a potassium atom with 19 protons and 20 neutrons
- a lithium atom with 3 protons and 4 neutrons
- Answer
-
- \(\ce{^{16}_8O}\)
- \(\ce{^{39}_19K}\)
- \(\ce{^{7}_3Li}\)
- Give complete symbols of each atom, including the atomic number and the mass number.
- a magnesium atom with 12 protons and 12 neutrons
- a magnesium atom with 12 protons and 13 neutrons
- a xenon atom with 54 protons and 77 neutron
- Answer
-
- \(\ce{^{24}_12Mg}\)
- \(\ce{^{25}_12Mg}\)
- \(\ce{^{131}_54Xe}\)
- Americium-241 is an isotope used in smoke detectors. What is the complete symbol for this isotope?
- Answer
-
\(\ce{^{241}_95Am}\)
- Carbon-14 is an isotope used to perform radioactive dating tests on previously living material. What is the complete symbol for this isotope?
- Answer
-
\(\ce{^{14}_6C}\)
- A rare isotope of helium has a single neutron in its nucleus. Write the complete atomic symbol of this isotope.
- Answer
-
\(\ce{^{3}_2He}\)
- Americium-241 is a crucial part of many smoke detectors. How many neutrons are present in its nucleus?
- Answer
-
146 neutrons
- Potassium-40 is a radioactive isotope of potassium that is present in the human body. How many neutrons are present in its nucleus?
- Answer
-
21 neutrons
- Give the number of protons, electrons, and neutrons in neutral atoms of each of the following isotopes:
- \(\ce{^{10}_5B}\)
- \(\ce{^{199}_{80}Hg}\)
- \(\ce{^{63}_{29}Cu}\)
- \(\ce{^{13}_6C}\)
- \(\ce{^{77}_{34}Se}\)
- Answer
-
- 5 protons, 5 electrons, 5 neutrons
- 80 protons, 80 electrons, 119 neutrons
- 29 protons, 29 electrons, 34 neutrons
- 6 protons, 6 electrons, 7 neutrons
- 34 protons, 34 electrons, 43 neutrons
- Give the number of protons, electrons, and neutrons in neutral atoms of each of the following isotopes:
- \(\ce{^7_3Li}\)
- \(\ce{^{125}_{52}Te}\)
- \(\ce{^{109}_{47}Ag}\)
- \(\ce{^{15}_7N}\)
- \(\ce{^{31}_{15}P}\)
- Answer
-
- 3 protons, 3 electrons, 4 neutrons
- 52 protons, 52 electrons, 73 neutrons
- 47 protons, 47 electrons, 62 neutrons
- 7 protons, 7 electrons, 8 neutrons
- 15 protons, 15 electrons, 16 neutrons
4.8: Atomic Mass
- Define atomic mass. What is its unit?
- Answer
-
Atomic mass is the average mass of atoms of an element. Its unit is atomic mass units (amu).
- Determine the atomic mass of each element, given the isotopic composition.
- lithium, which is 92.4% lithium-7 (mass 7.016 amu) and 7.60% lithium-6 (mass 6.015 amu)
- oxygen, which is 99.76% oxygen-16 (mass 15.995 amu), 0.038% oxygen-17 (mass 16.999 amu), and 0.205% oxygen-18 (mass 17.999 amu)
- Answer
-
- 6.94 amu
- 16.00 amu
- Determine the atomic mass of each element, given the isotopic composition.
- neon, which is 90.48% neon-20 (mass 19.992 amu), 0.27% neon-21 (mass 20.994 amu), and 9.25% neon-22 (mass 21.991 amu)
- uranium, which is 99.27% uranium-238 (mass 238.051 amu) and 0.720% uranium-235 (mass 235.044 amu)
- Answer
-
- 20.18 amu
- 238.0 amu
- An element has the following natural abundances and isotopic masses: 90.92% abundance with 19.99 amu, 0.26% abundance with 20.99 amu, and 8.82% abundance with 21.99 amu. Calculate the average atomic mass of this element.
- Answer
-
20.17 amu
- Average atomic masses listed by IUPAC are based on a study of experimental results. Bromine has two isotopes 79Br and 81Br, whose masses (78.9183 and 80.9163 amu) and abundances (50.69% and 49.31%) were determined in earlier experiments. Calculate the average atomic mass of bromine based on these experiments.
- Answer
-
79.90 amu
- Variations in average atomic mass may be observed for elements obtained from different sources. Lithium provides an example of this. The isotopic composition of lithium from naturally occurring minerals is 7.5% 6Li and 92.5% 7Li, which have masses of 6.01512 amu and 7.01600 amu, respectively. A commercial source of lithium, recycled from a military source, was 3.75% 6Li (and the rest 7Li). Calculate the average atomic mass values for each of these two sources.
- Answer
-
mineral = 6.94 amu; commercial = 6.978 amu
- The 18O:16O abundance ratio in some meteorites is greater than that used to calculate the average atomic mass of oxygen on earth. Is the average mass of an oxygen atom in these meteorites greater than, less than, or equal to that of a terrestrial oxygen atom?
- Answer
-
The average mass of an oxygen atom in the meteorites will be greater than the average mass of a terrestrial oxygen atom due to the extra 18O.
4.9: Ion Formation
- Explain how cations form.
- Answer
-
Cations are formed when an atom loses electrons.
- Explain how anions form.
- Answer
-
Anions are formed when an atom gains electrons.
- Give the charge each atom takes when it forms an ion.
- K
- O
- Answer
-
- K+
- O2−
- Give the charge each atom takes when it forms an ion.
- Ca
- I
- Answer
-
- Ca2+
- I−
- Give the charge each atom takes when it forms an ion.
- Al
- Br
- Answer
-
- Al3+
- Br−
- Give the charge each atom takes when it forms an ion.
- S
- Na
- Answer
-
- S2−
- Na+
- Write the symbol for each of the following ions:
- the ion with a 1+ charge, atomic number 55, and mass number 133
- the ion with 54 electrons, 53 protons, and 74 neutrons
- the ion with atomic number 15, mass number 31, and a 3− charge
- the ion with 24 electrons, 30 neutrons, and a 3+ charge
- Answer
-
- 133Cs+
- 127I−
- 31P3−
- 57Co3+
- Write the symbol for each of the following ions:
- the ion with a 3+ charge, 28 electrons, and a mass number of 71
- the ion with 36 electrons, 35 protons, and 45 neutrons
- the ion with 86 electrons, 142 neutrons, and a 4+ charge
- the ion with a 2+ charge, atomic number 38, and mass number 87
- Answer
-
71Ga3+
80Br−
232Th4+
87Sr2+
- Determine the number of protons, electrons, and neutrons in the following isotopes that are used in medical diagnoses:
- atomic number 9, mass number 18, charge of 1−
- atomic number 43, mass number 99, charge of 7+
- atomic number 53, atomic mass number 131, charge of 1−
- atomic number 81, atomic mass number 201, charge of 1+
- Answer
-
- 9 protons, 10 electrons, 9 neutrons
- 43 protons, 36 electrons, 56 neutrons
- 53 protons, 54 electrons, 78 neutrons
- 81 protons, 80 electrons, 120 neutrons
- The following are properties of isotopes of two elements that are essential in our diet. Determine the number of protons, electrons and neutrons in each and name them.
- atomic number 26, mass number 58, charge of 2+
- atomic number 53, mass number 127, charge of 1−
- Answer
-
- 26 protons, 24 electrons, 32 neutrons; iron(II) ion
- 53 protons, 54 electrons, 74 neutrons; iodide ion
This page was adapted from "Beginning Chemistry (Ball)" by LibreTexts and "Chemistry (OpenStax)" by LibreTexts and is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Vicki MacMurdo (Anoka-Ramsey Community College) and Lance S. Lund (Anoka-Ramsey Community College).


