2.11: Exercises
- Page ID
- 357317
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
2.1: Taking Measurements
- Identify the unit in each quantity.
- 2 boxes of crayons
- 3.5 grams of gold
- Answer
-
- boxes of crayons
- grams of gold
- Identify the unit in each quantity.
- 32 oz of cheddar cheese
- 0.045 cm3 of water
- Answer
-
- oz of cheddar cheese
- cm3 of water
- Identify the unit in each quantity.
- 9.58 s (the current world record in the 100 m dash)
- 6.14 m (the current world record in the pole vault)
- Answer
-
- s
- m
- Identify the unit in each quantity.
- 2 dozen eggs
- 2.4 km/s (the escape velocity of the moon, which is the velocity you need at the surface to escape the moon's gravity)
- Answer
-
- dozen eggs
- km/s
2.2: Scientific Notation
- Express these numbers in scientific notation.
- 56.9
- 563,100
- 0.0804
- 0.00000667
- Answer
-
- 5.69 × 101
- 5.631 × 105
- 8.04 × 10−2
- 6.67 × 10−6
- Express these numbers in scientific notation.
- −890,000
- 602,000,000,000
- 0.0000004099
- 0.000000000000011
- Answer
-
- −8.9 × 105
- 6.02 × 1011
- 4.099 × 10−7
- 1.1 × 10−14
- Express these numbers in scientific notation.
- 0.00656
- 65,600
- 4,567,000
- 0.000005507
- Answer
-
- 6.56 × 10−3
- 6.56 × 104
- 4.567 × 106
- 5.507 × 10−6
- Express these numbers in scientific notation.
- 65
- −321.09
- 0.000077099
- 0.000000000218
- Answer
-
- 6.5 × 101
- −3.2109 × 102
- 7.7099 × 10−5
- 2.18 × 10−10
- Express these numbers in decimal notation.
- 1.381 × 105
- 5.22 × 10−7
- 9.998 × 104
- Answer
-
- 138,100
- 0.000000522
- 99,980
- Express these numbers in decimal notation.
- 7.11 × 10−2
- 9.18 × 102
- 3.09 × 10−10
- Answer
-
- 0.0711
- 918
- 0.000000000309
- Express these numbers in decimal notation.
- 8.09 × 100
- 3.088 × 10−5
- −4.239 × 102
- Answer
-
- 8.09
- 0.00003088
- −423.9
- Express these numbers in decimal notation.
- 2.87 × 10−8
- 1.78 × 1011
- 1.381 × 10−23
- Answer
-
- 0.0000000287
- 178,000,000,000
- 0.00000000000000000000001381
2.3: Significant Figures
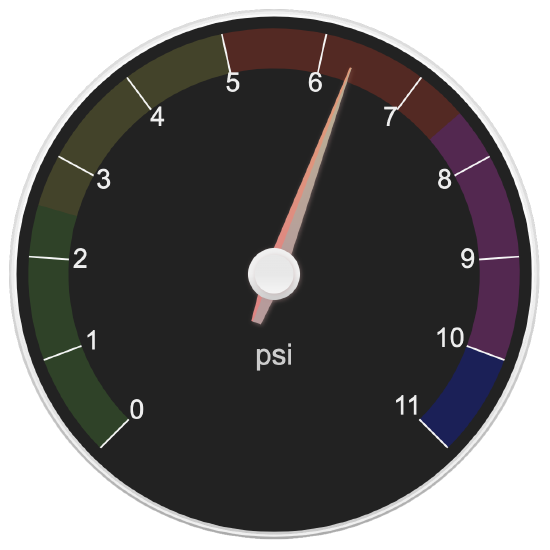
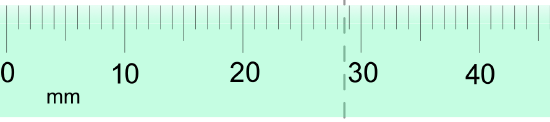
- Express each measurement to the correct number of significant figures.
- Answer
-
- 6.3 psi
- 28.6 mm
- 477 psi
- 35 mm
- How many significant figures do these numbers have?
- 23
- 23.0
- 0.00023
- 0.0002302
- Answer
-
- 2
- 3
- 2
- 4
- How many significant figures do these numbers have?
- 5.44 × 108
- 1.008 × 10−5
- 43.09
- 0.0000001381
- Answer
-
- 3
- 4
- 4
- 4
- How many significant figures do these numbers have?
- 765,890
- 765,890.0
- 1.2000 × 105
- 0.0005060
- Answer
-
- 5 or 6, ambiguous
- 7
- 5
- 4
- How many significant figures do these numbers have?
- 0.009
- 0.0000009
- 65,444
- 65,040
- Answer
-
- 1
- 1
- 5
- 4 or 5, ambiguous
- Write the number 87,449 in scientific notation with four significant figures.
- Answer
-
8.745 × 104
- Write the number 0.000066600 in scientific notation with five significant figures.
- Answer
-
6.6600 × 10−5
- Write the number 0.0000558 in scientific notation with two significant figures.
- Answer
-
5.6 × 10−5
2.4: Significant Figures in Calculations
- Compute and express each answer with the proper number of significant figures, rounding as necessary.
- 56.0 + 3.44 = ?
- 0.00665 + 1.004 = ?
- 45.99 − 32.8 = ?
- 45.99 − 32.8 + 75.02 = ?
- Answer
-
- 59.4
- 1.011
- 13.2
- 88.2
- Compute and express each answer with the proper number of significant figures, rounding as necessary.
- 1.005 + 17.88 = ?
- 5,670.0 − 324 = ?
- 405,007 − 123.3 = ?
- 55.5 + 66.66 − 77.777 = ?
- Answer
-
- 18.89
- 5,346
- 404,884
- 44.4
- Compute and express each answer with the proper number of significant figures, rounding as necessary.
- 56.7 × 66.99 = ?
- 1.00 ÷ 77 = ?
- 1.00 ÷ 77.0 = ?
- 6.022 × 1.89 = ?
- Answer
-
- 3.80 × 103
- 0.013
- 0.0130
- 11.4
- Compute and express each answer with the proper number of significant figures, rounding as necessary.
- 0.000440 × 17.22 = ?
- 203,000. ÷ 0.044 = ?
- 67 × 85.0 × 0.0028 = ?
- 999,999 ÷ 33.1 = ?
- Answer
-
- 0.00758
- 4.6 × 106
- 16
- 3.02 × 104
- Compute and express each answer with the proper number of significant figures, rounding as necessary.
- 67,883 × 0.004321 = ?
- (9.67 × 103) × 0.0055087 = ?
- Answer
-
- 293.3
- 53.3
- Compute and express each answer with the proper number of significant figures, rounding as necessary.
- 18,900. × 76.33 ÷ 0.00336 = ?
- 0.77604 ÷ 76,003 × 8.888 = ?
- Answer
-
- 4.29 × 108
- 9.075 × 10−5
2.5: The Metric System
- Indicate what multiplier each prefix represents.
- k
- m
- M
- Answer
-
- 103 or 1,000 ×
- 10−3 or \(\frac1{1000}\) ×
- 106 or 1,000,000 ×
- Indicate what multiplier each prefix represents.
- c
- G
- μ
- Answer
-
- 10−2 or \(\frac1{100}\) ×
- 109 or 1,000,000,000 ×
- 10−6 or \(\frac1{1,000,000}\) ×
- Give the prefix that represents each multiplier.
- 10−3 or \(\frac1{1000}\) ×
- 103 or 1,000 ×
- 109 or 1,000,000,000 ×
- Answer
-
- m or milli
- k or kilo
- G or giga
- Give the prefix that represents each multiplier.
- \(\frac1{1,000,000,000}\) ×
- \(\frac1{100}\) ×
- 1,000,000 ×
- Answer
-
- n or nano
- c or centi
- M or mega
- Express each quantity in a more appropriate unit. There may be more than one acceptable answer.
- 3.44 × 10−9 s
- 3,500 L
- 0.045 m
- Answer
-
- 3.44 ns
- 3.5 kL
- 45 mm
- Express each quantity in a more appropriate unit. There may be more than one acceptable answer.
- 0.000066 m/s (Hint: you need consider only the unit in the numerator.)
- 4.66 × 106 s
- 7,654 L
- Answer
-
- 66 μm/s
- 4.66 Ms
- 7.654 kL
- Express each quantity in a more appropriate unit. There may be more than one acceptable answer.
- 43,600 mL
- 0.0000044 m
- 1,438 ms
- Answer
-
- 43.6 L
- 4.4 μm
- 1.438 s
2.6: Problem Solving and Unit Conversions
- Write the two conversion factors that exist between the two given units.
- milliliters and liters
- nanoseconds and seconds
- kilometers and meters
- Answer
-
- \[ \frac{\text{1000 mL}}{\text{1 L}} {\text{ and }} \frac{\text{1 L}}{\text{1000 mL}}\nonumber\]
- \[ \frac{\text{1,000,000,000 ns}}{\text{1 s}} {\text{ and }} \frac{\text{1 s}}{\text{1,000,000,000 ns}}\nonumber\]
- \[ \frac{\text{1 km}}{\text{1000 m}} {\text{ and }} \frac{\text{1000 m}}{\text{1 km}}\nonumber\]
- Write the two conversion factors that exist between the two given units.
- kilograms and grams
- milliseconds and seconds
- centimeters and meters
- Answer
-
- \[\frac{\text{1 kg}}{\text{1000 g}} {\text{ and }} \frac{\text{1000 g}}{\text{1 kg}}\nonumber\]
- \[\frac{\text{1000 ms}}{\text{1 s}} {\text{ and }} \frac{\text{1 s}}{\text{1000 ms}}\nonumber\]
- \[\frac{\text{100 cm}}{\text{1 m}} {\text{ and }} \frac{\text{1 m}}{\text{100 cm}}\nonumber\]
- Perform the following conversions.
- 5.4 km to meters
- 0.665 m to millimeters
- 0.665 m to kilometers
- Answer
-
- 5,400 m
- 665 mm
- 6.65 × 10−4 km
- Perform the following conversions.
- 90.6 mL to liters
- 0.00066 ML to liters
- 750 L to kiloliters
- Answer
-
- 0.0906 L
- 660 L
- 0.75 kL
- Perform the following conversions.
- 17.8 μg to grams
- 7.22 × 102 kg to grams
- 0.00118 g to nanograms
- Answer
-
- 1.78 × 10−5 g
- 7.22 × 105 g
- 1.18 × 106 ng
- Perform the following conversions.
- 833 ns to seconds
- 5.809 s to milliseconds
- 2.77 × 106 s to megaseconds
- Answer
-
- 8.33 × 10−7 s
- 5,809 ms
- 2.77 Ms
- Perform the following conversions.
- 45.0 m/min to meters/second
- 0.000444 m/s to micrometers/second
- 60.0 km/h to kilometers/second
- Answer
-
- 0.750 m/s
- 444 μm/s
- 0.0167 km/s
- Perform the following conversions.
- 3.4 × 102 cm/s to centimeters/minute
- 26.6 mm/s to millimeters/hour
- 13.7 kg/L to kilograms/milliliters
- Answer
-
- 2.0 × 104 cm/min
- 9.58 × 104 mm/h
- 0.0137 kg/mL
2.7: Solving Multi-Step Conversion Problems
- Perform the following conversions.
- 0.674 kL to milliliters
- 2.81 × 1012 mm to kilometers
- 94.5 kg to milligrams
- Answer
-
- 6.74 × 105 mL
- 2.81 × 106 km
- 9.45 × 107 mg
- Perform the following conversions.
- 6.79 × 10−6 kg to micrograms
- 1.22 mL to kiloliters
- 9.508 × 10−9 ks to milliseconds
- Answer
-
- 6.79 × 103 μg
- 1.22 × 10−6 kL
- 9.508 × 10−3 ms
- Perform the following conversions.
- 6.77 × 1014 ms to kiloseconds
- 34,550,000 cm to kilometers
- Answer
-
- 6.77 × 108 ks
- 345.5 km
- Perform the following conversions.
- 4.701 × 1015 mL to kiloliters
- 8.022 × 10−11 ks to microseconds
- Answer
-
- 4.701 × 109 kL
- 0.08022 μs
- Perform the following conversions. Note that you will have to convert units in both the numerator and the denominator.
- 88 ft/s to miles/hour
- 0.00667 km/h to meters/second
- Answer
-
- 6.0 × 101 mi/h
- 1.85 × 10−3 m/s
- Perform the following conversions. Note that you will have to convert units in both the numerator and the denominator.
- 3.88 × 102 mm/s to kilometers/hour
- 1.004 kg/L to grams/milliliter
- Answer
-
- 1.40 km/h
- 1.004 g/mL
2.8: Units Raised to a Power
- Perform the following conversions.
- 9.44 m2 to square centimeters
- 3.44 × 108 mm3 to cubic meters
- Answer
-
- 9.44 × 104 cm2
- 0.344 m3
- Perform the following conversions.
- 0.00444 cm3 to cubic meters
- 8.11 × 102 m2 to square nanometers
- Answer
-
- 4.44 × 10−9 m3
- 8.11 × 1020 nm2
- Why would it be inappropriate to convert square centimeters to cubic meters?
- Answer
-
One is a unit of area and the other is a unit of volume.
- The formula for the area of a triangle is ½ × base × height. What is the area of a triangle in square meters if its base is 166 mm and its height is 930.0 mm? Express the answer to the proper number of significant figures.
- Answer
-
0.0772 m2
- What is the area in square millimeters of a rectangle whose sides are 2.44 cm × 6.077 cm? Express the answer to the proper number of significant figures.
- Answer
-
1.48 × 103 mm2
- What is the volume in cubic centimeters of a cube with sides of 0.774 m? Express the answer to the proper number of significant figures.
- Answer
-
4.64 × 105 cm3
- The formula for the area of a triangle is ½ × base × height. What is the area of a triangle in square centimeters if its base is 1.007 m and its height is 0.665 m? Express the answer to the proper number of significant figures.
- Answer
-
3.35 × 103 cm2
2.9: Density
- A block of metal alloy has a mass of 34.96 g. Its dimensions are 3.9 cm by 4.2 cm by 1.6 cm. What is the density of the metal alloy?
- Answer
-
1.3 g/cm3
- A plastic cylinder with a mass of 26.7 g is added to a graduated cylinder containing 45.8 mL of water. Once the cylinder was submerged, the volume increased to 61.3 mL. What was the density of the plastic cylinder?
- Answer
-
1.72 g/mL
- A sample of iron has a volume of 48.2 cm3. If the density of iron is 7.87 g/cm3, what is its mass?
- Answer
-
379 g
- A sample of air has a volume of 1,015 mL. What is its mass? Consult Table 2.9.1 for the density.
- Answer
-
1.22 g
- The volume of hydrogen used by the Hindenburg, the German airship that exploded in New Jersey in 1937, was 2.000 × 108 L. What mass of hydrogen was used by the airship? Consult Table 2.9.1 for the density.
- Answer
-
1.7 × 107 g
- The volume of an Olympic-sized swimming pool is 2.50 × 109 cm3. If the pool is filled with alcohol (d = 0.789 g/cm3), what mass of alcohol is in the pool?
- Answer
-
1.97 × 109 g
- A typical engagement ring has 0.77 cm3 of gold. What mass of gold is present? Consult Table 2.9.1 for the density.
- Answer
-
15 g
- A typical mercury thermometer has 0.039 mL of mercury in it. What mass of mercury is in the thermometer? Consult Table 2.9.1 for the density.
- Answer
-
0.53 g
- What is the volume of 100.0 g of lead? Consult Table 2.9.1 for the density.
- Answer
-
8.811 cm3
- What is the volume of 255.0 g of uranium if uranium has a density of 19.05 g/cm3?
- Answer
-
13.39 cm3
- What is the volume in liters of 222 g of neon if neon has a density of 0.900 g/L?
- Answer
-
247 L
- What is the volume in liters of 20.5 g of sulfur hexafluoride if sulfur hexafluoride has a density of 6.164 g/L?
- Answer
-
3.33 L
- Which has the greater volume, 100.0 g of iron (d = 7.87 g/cm3) or 75.0 g of gold (d = 19.3 g/cm3)?
- Answer
-
Iron. The volume of the iron is 12.7 cm3 and the volume of the gold is 3.89 cm3.
- Which has the greater volume, 25.0 g of hydrogen gas (d = 0.0840 g/L) or 100.0 g of argon gas (d = 1.78 g/L)?
- Answer
-
Hydrogen. The volume of the hydrogen is 298 L and the volume of the argon is 56.2 L.
Additional Exercises
- Evaluate 0.00000000552 × 0.0000000006188 and express the answer in scientific notation. You may have to rewrite the original numbers in scientific notation first.
- Answer
-
3.42 × 10−18
- Evaluate 333,999,500,000 ÷ 0.00000000003396 and express the answer in scientific notation. You may need to rewrite the original numbers in scientific notation first.
- Answer
-
9.835 × 1021
- Fill in the blank: 1 km = ______________ μm.
- Answer
-
109 μm
- Fill in the blank: 1 Ms = ______________ ns.
- Answer
-
1015 ns
- Fill in the blank: 1 cL = ______________ ML.
- Answer
-
10−8 ML
- Fill in the blank: 1 mg = ______________ kg.
- Answer
-
10−6 kg
- Convert a speed of 60.0 mi/h into kilometers/hour.
- Answer
-
96.5 km/h
- Convert a speed of 60.0 km/h into miles/hour.
- Answer
-
37.3 mi/h
- Convert 52.09 km/h into meters/second.
- Answer
-
14.47 m/s
- Convert 2.155 m/s into kilometers/hour.
- Answer
-
7.758 km/h
- What is the mass of 12.67 L of mercury? Consult Table 2.9.1 for the density.
- Answer
-
1.72 × 105 g
- What is the mass of 0.663 m3 of air? Consult Table 2.9.1 for the density.
- Answer
-
796 g
- What is the volume of 2.884 kg of gold? Consult Table 2.9.1 for the density.
- Answer
-
149 cm3
- What is the volume of 40.99 kg of cork? Assume a density of 0.22 g/cm3.
- Answer
-
1.9 × 105 cm3
This page was adapted from "Beginning Chemistry (Ball)" by LibreTexts and is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Vicki MacMurdo (Anoka-Ramsey Community College) and Lance S. Lund (Anoka-Ramsey Community College).