10.1: Nucleotides - The Building Blocks of Nucleic Acids
- Page ID
- 433036
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe the makeup of nucleotides that are building blocks of nucleic acids DNA and RNA.
Nucleic acids are biopolymers that carry the codes for the synthesis of proteins and pass them on from generation to generation, i.e., they are genetic materials. In other words, nucleic acids are the instruction manual for biochemical reactions taking place in living things.
Nucleotides are the building blocks, i.e., the repeat units or monomers of nucleic acids.
Nucleotides are composed of three sub-units:
- a 5-carbon carbohydrate,
- a base that is an aromatic compound containing nitrogen, and
- an anion of phosphoric acid, i.e., phosphate (\(\ce{PO4^{3-}}\)).
5-Carbon Monosaccharide
There are two 5-carbon carbohydrates i.e., ribose and deoxyribose, found in nucleic acids, as shown in the figure 10.1.1 below. Both are in furanose (five-membered cyclic) form with hydroxyl (\(\ce{-OH}\)) group at anomeric (\(\ce{C}\)#1') in a \(\beta\) configuration. The number labels have prime symbols on them, i.e. 1', 2', 3' etc. to distinguish them from regular numbers 1, 2, 3, etc that are used for the nitrogen bases of the nucleic acids. The only difference between ribose and deoxyribose is that hydroxy group at \(\ce{C}\)#2' is missing in the latter, which gives the deoxy prefix to its name.

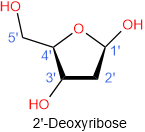
Figure \(\PageIndex{1}\): The two monosaccharides in nucleic acids.
There are two types of nucleic acids, one contains ribose in its nucleotides is called ribonucleic acid (RNA) and the other contains deoxyribose in its nucleotides is called deoxyribonucleic acid (DNA).
Nitrogen bases
Nitrogen bases in nucleic acids are derivatives of two aromatic compounds; purine and pyrimidine, shown in figure 10.1.2.


Figure \(\PageIndex{2}\): Purine and Pyrimidine.
Cyclic compounds that contain atoms other than \(\ce{C's}\) in the cycle are called heterocyclic compounds. Purine and pyrimidine are heterocyclic aromatic compounds because they contain \(\ce{N's}\) in the cycles. Purine is bicyclic containing a six-membered ring with two \(\ce{N's}\) fused with a five-membered ring with two \(\ce{N's}\). Pyrimidine is a six-membered cycle with two \(\ce{N's}\). Both purine and pyrimidine are planar molecules like benzene.
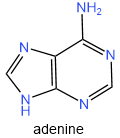
There are five nitrogen bases found in nucleic acids: two are purines, i.e., adenine (A) and guanine (G), and three are pyrimidines, i.e., cytosine (C), thymine (T), and uracil (U), as shown in figure 10.1.3.
| Purines | Pyrimidines | |||
|---|---|---|---|---|
 |
 |
 |
 |
 |
Figure \(\PageIndex{3}\): The five bases in nucleic acids.
DNA contains four nitrogen bases, i.e., adenine, guanine, cytosine, and thymine. RNA also contains four nitrogen bases i.e., adenine, guanine, cytosine, and uracil. Note that the first three, i.e., adenine, guanine, and cytosine are common in DNA and RNA, but the fourth, i.e, thymine in DNA is replaced with uracil in RNA.
Nucleosides
When a monomer sugar like ribose or deoxyribose reacts with an amine, the \(\ce{-OH}\) group at \(\ce{C}\)#1' is replaced with a \(\ce{N}\) of the amine. The product is called N-glycoside, and the \(\ce{C-N}\) bond in the N-glycoside is called an N-glycosidic bond, as shown in the following example.

Figure \(\PageIndex{4}\):Purines and pyrimidines connect with the anomeric carbon of the sugar to form the N-glycoside.
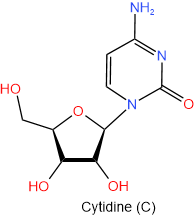
The N-glycosides are named by using the name of purine but ending with -osine e.g., adenine becomes adenosine and guanine becomes gunosine, or by using the name of pyrimidine ending with -idine, e.g., cytosine becomes cytidine and uracil become uridine. One letter abbreviation of the nitrogen bases remains the same for the corresponding nucleoside, e.g., adenine (A) and adenosine (A). The four nucleosides found in RNA are shown in figure 10.1.5 with their names and one-letter abbreviations.
 |
 |
 |
 |
Figure \(\PageIndex{5}\): The four nucleosides of RNA.
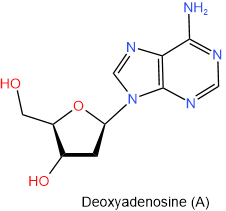
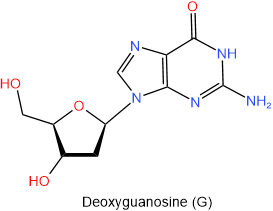
In DNA there is deoxyribose in place of ribose. So the names of nucleosides found in DNA begin with the deoxy- prefix. The four nucleosides found in DNA are shown in figure 10.1.6 with their names and one-letter abbreviations.
 |
 |
 |
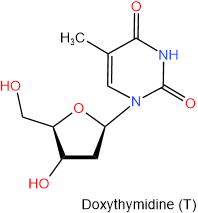 |
Figure \(\PageIndex{6}\): The four nucleosides of DNA.
Phosphate
Phosphoric acid (H3PO4) is an oxyacid with three acidic protons, i.e., it is triprotic acid. Two phosphoric acids condense and form diphosphoric acid, with the elimination of water.
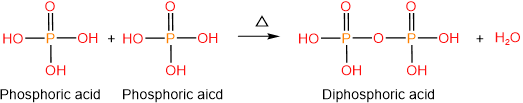
Similarly, three phosphoric acids can condense to form triphosphoric acid. Phosphoric acid, diphosphoric acid, and triphosphoric acid are shown below.



Under physiological conditions at pH ~7.4, the phosphoric acids loose proton and exist as phosphate anions as shown below.

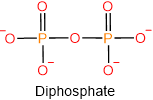

Phosphoric acids also condense with alcohols (\(\ce{R-OH}\)) and form mono-, di-, or triesters, as illustrated below.



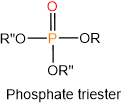
Nucleotide
Phosphate esters of nucleosides are called nucleotides. The nucleotides found in nucleic acids are formed when \(\ce{-OH}\) group at \(\ce{C}\)#5 of ribose or deoxyribose makes ester with a phosphate. Examples of monphosphate, diphosphate, and triphosphate esters of adenosine are shown in figure 10.1.7.



Figure \(\PageIndex{7}\):Phosphate esters of nucleosides are called nucleotides.
The names of the nucleotides are derived by using the name of the corresponding nucleoside with monophosphate, diphosphate, or triphosphate added at the end, depending on whether the phosphate group is monophosphate, diphosphate, or triphosphate. Abbreviation of the nucleotide starts with the one-letter abbreviation of the nitrogen base followed by MP, DP, or TP depending on whether the phosphate group is monophosphate, diphosphate, or triphosphate, as shown in the example structures above.
The nucleotides like ADP, and ATP are high-energy molecules that are energy-rich molecules in biochemical systems. For example, when ATP converts to ADP by releasing a phosphate, energy is released that is used to do work or to synthesize other compounds that need energy for the synthesis. Synthesis of nucleic acids begins with triphosphate esters that convert into monophosphate esters when they incorporate in the nucleic acid polymer. The energy released in the conversion of triphosphate to monophosphate is used to power an endothermic reaction.
The four nucleotides found in RNA are shown in figure 10.8.1 with their names and abbreviations.
 ,
,  ,
,  , and
, and 
Figure \(\PageIndex{8}\): The four nucleotides found in RNA.
The nucleotides found in DNA are named similarly to those of RNA, i.e., start with the name of the corresponding nucleoside and end with monophosphate, diphosphate, or triphosphate. The abbreviations of the nucleotides found in DNA start with a small alphabet d representing the deoxy- prefix of the nucleotides. The four nucleotides found in DNA are shown in figure 10.1.9 with their names and abbreviations.
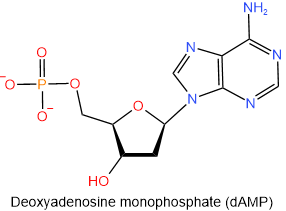 ,
, 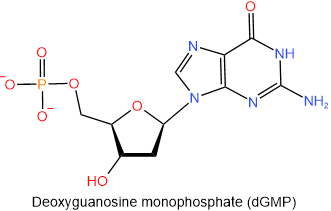 ,
,  ,
, 
Figure \(\PageIndex{9}\): The four nucleotides found in DNA.
Summary
Nucleotides are composed of an anion of phosphoric acid, a pentose sugar (ribose or deoxyribose), and a nitrogen-containing base (adenine, cytosine, guanine, thymine, or uracil). Ribonucleotides contain ribose, while deoxyribonucleotides contain deoxyribose.


