9.3: The Peptide Bond
- Page ID
- 433003
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain how a peptide bond is formed from individual amino acids.
- Identify the N-terminus and C-terminus of a polypeptide or protein.
Our body has an intricate mechanism for the manufacture of proteins. Scientists have to use other techniques in order to synthesize the same proteins in a lab. The chemistry of peptide synthesis is complicated. Both active groups on an amino acid can react and the amino acid sequence must be a specific one in order for the protein to function. Robert Merrifield developed the first synthetic approach for making proteins in the lab, a manual approach which was lengthy and tedious (and, he won the Nobel Prize in Chemistry in 1984 for his work). Today, however, automated systems can crank out a peptide in a very short period of time.
The Peptide Bond
A peptide bond is formed by a combination of amino acids in which the amine group of one amino acid has undergone a reaction with the carboxylic acid of another amino acid. The reaction is a dehydration-condensation reaction, forming an amide group \(\left( \ce{CO-NH} \right)\), shown below. The result is elimination of a water molecule and condensation of two amino acids. The molecule formed is called a dipeptide.
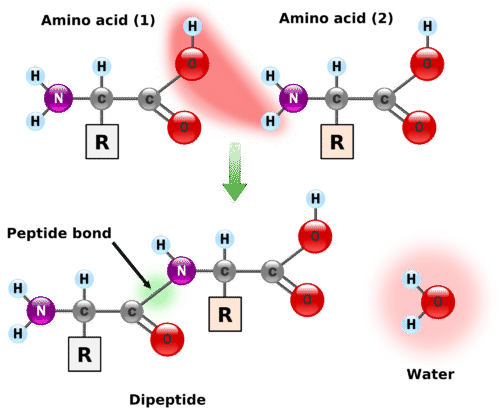
A chain consisting of only two amino acid units is called a dipeptide; a chain consisting of three is a tripeptide. By convention, peptide and protein structures are depicted with the amino acid whose amino group is free (the N-terminal end) on the left and the amino acid with a free carboxyl group (the C-terminal end) to the right as shown in figure 9.3.2.

Figure \(\PageIndex{2}\): A tripeptide showing the two terminus.
The sequence of amino acids in a chain is called an amino acid sequence. Suppose that a sequence of the amino acids glycine, tryptophan, and alanine is formed with the free amine group as part of the glycine and the free carboxyl group as part of the alanine. The amino acid sequence can be easily written using the abbreviations as Gly-Trp-Ala. This is a different sequence from Ala-Trp-Gly because the free amine and carboxyl groups would be on different amino acids in that case. Gly-Trp-Ala and Ala-Trp-Gly are isomers (figure 9.3.3) and have different biological functions and properties.
Figure \(\PageIndex{3}\): Two tripeptides that are isomers of each other.
The general term polypeptide refers to an amino acid chain of unspecified length. However, chains of about 50 amino acids or more are usually called proteins. In its physiologically active form, a protein may be composed of one or more polypeptide chains.
Draw the polypeptide Asp-Val-Ser.
Solution
1. Identify the structures of each of the three given amino acids and draw them in the same order as given in the name.
2. Leaving the order the same, connect the amino acids to one another by forming peptide bonds. Note that the order given in the name is the same way the amino acids are connected in the molecule. The first one listed is always the \(\ce{N}\)-terminus of the polypeptide.
List all of the possible polypeptides that can be formed from cysteine (Cys), leucine (Leu), and arginine (Arg).
Solution
Although there are only three amino acids, the order in which they are bonded changes the identity, properties, and function of the resulting polypeptide. There are six possible polypeptides formed from these three amino acids.
Cys-Leu-Arg
Cys-Arg-Leu
Leu-Cys-Arg
Leu-Arg-Cys
Arg-Cys-Leu
Arg-Leu-Cys
Contributors and Attributions
Allison Soult, Ph.D. (Department of Chemistry, University of Kentucky)





