8.10: Compensation of pH via Organs
- Page ID
- 432867
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe the role of lungs and the kidneys in maintaining a stable blood serum pH with the buffers
The pH of your blood normally ranges between 7.35 and 7.45. A blood pH below the normal range is called acidosis. A blood pH above this range is called alkalosis. Either one is potentially fatal. Why? Enzymes catalyze chemical reactions in the body. The enzymes are made of proteins which are chains of amino acids connected via peptide bonds. Some of the amino acids in the proteins contain acidic or basic functional groups. Changes in the pH of the blood serum can change the nature of the functional groups in the side chain through neutralization reactions. This in turn will change the intermolecular forces that affect protein folding or shape of the protein. The change in the shape of the protein will adversely affect the function of the enzyme or protein.
To maintain a constant serum pH the body relies on two approaches.
- One involves the use of buffers to minimize the changes in hydronium ion concentrations. This was discussed in the earlier section 8.9.
- The other is to control the hydronium ion concentrations by the involvement of the organs such as lungs and the kidneys.
Carbonic Acid and Bicarbonate Ion Buffer System
The most important buffer system in blood is formed from carbonic acid (H2CO3) and its conjugate base, the bicarbonate ion (HCO3-). Carbon dioxide (CO2) is a product of metabolism. Some of the CO2 dissolves in the blood serum to generate carbonic acid as shown in equation 1. The carbonic acid then acts as a Bronsted-Lowry acid to set up an equilibrium as shown in equation 2.
\[\ce{H2O(l) + CO2(g) <=> H2CO3(aq)} \tag{1}\]
\[\ce{H2CO3(aq) + H2O(l) <=> HCO^{-}3(aq) + H3O^{+}(aq)} \tag{2}\]
Acidosis
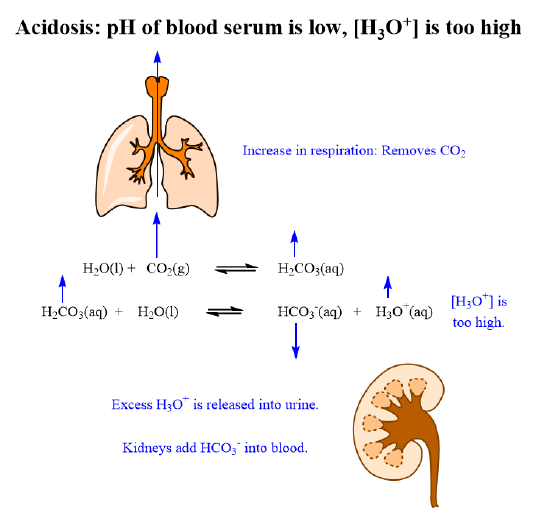
During conditions of acidosis, when the pH falls below 7.35, the concentration of H3O+ increases, the equilibrium in equation 2 shifts left increasing the concentration of H2CO3. Equilibrium in equation 1 then shifts left as a result of increasing H2CO3 concentrations, increasing the concentration of CO2. The CO2 is then expelled out by increased respiration to lower CO2. This is how the lungs compensate for acidosis.
The kidney play, a role during conditions of acidosis, when the pH falls below 7.35. As the concentration of H3O+ increases, the equilibrium in the equation shifts left, decreasing the concentration of HCO3- ion. The kidneys can then remove the excess H3O+ into the urine or add HCO3- ion into the blood stream, thus correcting the pH. This is how the kidneys compensate for acidosis.
The role of the organs to compensate for acidosis are summarized in figure 8.10.1 below.
Alkalosis
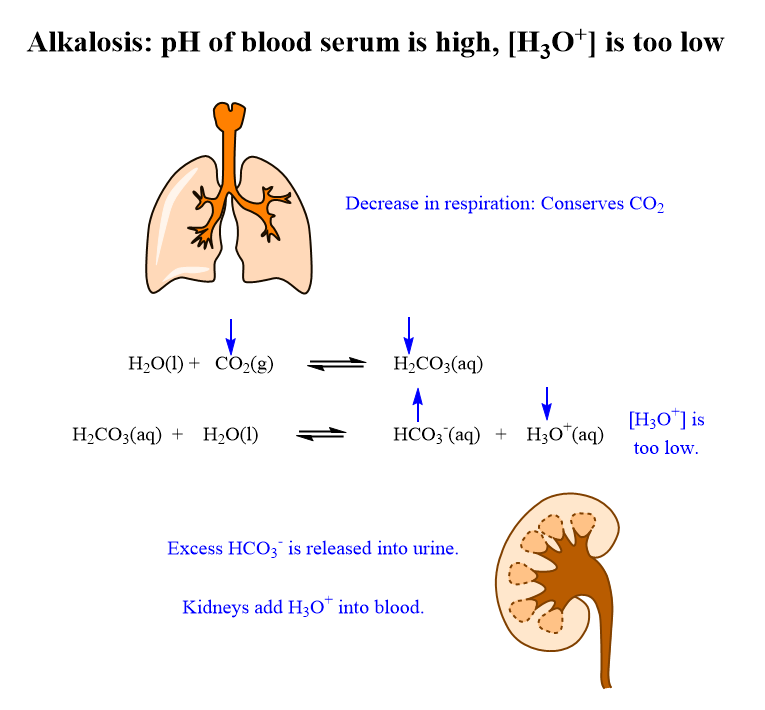
During conditions of alkalosis, when the pH increases above 7.45, the relative concentration of H3O+ decreases, the equilibrium in equation 2 shifts right decreasing the concentration of H2CO3. When the concentration of H2CO3 decreases the equilibrium in equation 1 shifts right decreasing the concentration of CO2. The respiration is then decreased to conserve CO2. This is compensation for alkalosis by the lungs.
\[\ce{ H2O(l) + CO2(g) <=> H2CO3(aq)}\tag{1}\]
\[\ce{H2CO3(aq) + H2O(l) <=> HCO^{-}3(aq) + H3O^{+}(aq)} \tag{2}\]
The kidney play, a role during conditions of alkalosis, when the pH is above 7.35. As the concentration of H3O+ decreases, the equilibrium in equation 2 shifts right increasing the concentration of HCO3- ion. The kidneys can then add H3O+ into the blood stream and remove excess the HCO3- ion through the urine, correcting the pH. This is compensation for alkalosis by the kidneys.
The role of the organs to compensate for alkalosis are summarized in figure 8.10.2 below.
Dihydrogenphosphate and the Hydrogenphosphate Buffer System
The second buffer system is the dihydrogenphosphate (H2PO4-) and its conjugate base the (HPO42-). The mixture of anions H2PO4- / HPO42- sets up the following acid-base equilibrium. Note the absence of gases in this equilibrium and the presence of three ions in the equilibrium.
\[\ce{H2PO^{-}4(aq) + H2O(l) ⇌ HPO^{2-}4(aq) + H3O^{+}(aq)} \nonumber\]
Acidosis
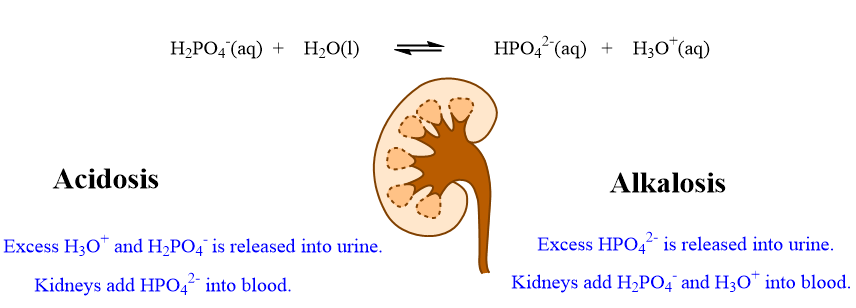
During conditions of acidosis, when the pH falls below 7.35, the concentration of H3O+ increases, the equilibrium in equation shifts left increasing the concentration of H2PO4- and decreasing the concentrations of HPO42-. The kidneys remove excess H3O+ and H2PO4- via urine and add HPO42- to the blood. This is another compensation for acidosis by the kidneys using a second buffer system.
Alkalosis
During conditions of alkalosis, when the pH is above 7.45, the relative concentration of H3O+ decreases, the equilibrium in the equation shifts right increasing the concentration of HPO42- ion and decreasing the concentrations of H2PO4- ion. The kidneys can then add H3O+ and H2PO4- into the blood stream and remove excess the HPO42- ion through the urine, correcting the pH. This is compensation for alkalosis by the kidneys.
The role of the kidneys to compensate for acidosis and alkalosis are summarized in the diagram below.
Even with all these controls, the pH of blood can still sometimes fall outside the normal range. The acid/base disorders are classified as being either respiratory or metabolic in nature. Respiratory disorders occur when exhaling does not remove CO2 from the blood at the same rate that it is produced in cells. Metabolic disorders arise from the inability to remove acids produced by metabolism, inability to control concentrations of HCO3-, ingestion of compounds that are acids or bases or compounds that can be converted to acids or bases. The following lists conditions that can lead to either acidosis or alkalosis.
Acidosis:
- Lung disease, asthma
- Excessive alcohol consumption
- Ketosis, ketoacidosis (starvation, diabetic conditions)
- Holding your breath too long
- Hypoventilation (not enough CO2 is exhaled)
Alkalosis:
- Excessive use of antacids
- Anxiety, fever
- Hyperventilation (CO2 is blown off faster than it is produced in the cells)
Mild cases of acidosis can lead to light-headedness. Severe cases can lead to coma and death. Symptoms of alkalosis include headaches, nervousness, cramps. In severe cases of alkalosis convulsions and death results.


