8.4: Brønsted-Lowry Theory of Acids and Bases
- Page ID
- 432654
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Identify a Brønsted-Lowry acid and a Brønsted-Lowry base.
- Understand amphoteric properties of ions and compounds.
- Identify conjugate acid-base pairs in an acid-base reaction.
There are three major theories of acids or bases. The theory developed by Svante Arrhenius in 1883, the Arrhenius definition, states that an acid produces H+ in solution and a base produces OH-. Later, two more sophisticated and general theories were proposed. These theories are the Brønsted-Lowry and Lewis definitions of acids and bases. This section covers the Brønsted-Lowry theory.
The Brønsted-Lowry Theory of Acids and Bases
In 1923, Danish chemist Johannes Brønsted and English chemist Thomas Lowry independently proposed new definitions for acids and bases, ones that focus on proton transfer. A Brønsted-Lowry acid is any species that can donate a proton (H+) to another molecule. A Brønsted-Lowry base is any species that can accept a proton from another molecule. In short, a Brønsted-Lowry acid is a proton donor, while a Brønsted-Lowry base is a proton acceptor.
A Brønsted-Lowry acid is a proton donor, while a Brønsted-Lowry base is a proton acceptor.
Example 1:
Let us use the reaction of ammonia in water to demonstrate the Brønsted-Lowry definitions of an acid and a base. Ammonia and water molecules are reactants, while the ammonium ion and the hydroxide ion are products:
\[\ce{NH3(aq) + H2O (ℓ) <=> NH^{+}4(aq) + OH^{−}(aq) }\label{Eq1}\]
What has happened in this reaction is that the original water molecule has donated a hydrogen ion to the original ammonia molecule, which in turn has accepted the hydrogen ion. We can illustrate this as follows:

Because the water molecule donates a hydrogen ion to the ammonia, it is the Brønsted-Lowry acid, while the ammonia molecule accepts the hydrogen ion, is the Brønsted-Lowry base.
Example 2:
In this example water and HCN are reactants, while cyanide ion and hydronium ion are products.
HCN(aq)+H2O(ℓ)  CN-(aq)+H3O+(aq)
CN-(aq)+H3O+(aq)
The water molecule has accepted a hydrogen ion from the HCN molecule which in turn donates a proton to water. Because the water molecule accepts a hydrogen ion from HCN, it is the Brønsted-Lowry base, while the HCN molecule donates the hydrogen ion is the Brønsted-Lowry acid.
The Hydronium Ion
Recall that the hydrogen atom is a single proton surrounded by a single electron. To make the hydrogen ion, we remove the electron, leaving a bare proton. Do we really have bare protons floating around in aqueous solution? No, we do not. What really happens is that the H+ ion attaches itself to H2O to make H3O+, which is called the hydronium ion. For most purposes, H+ and H3O+ represent the same species, but writing H3O+ instead of H+ shows that we understand that there are no bare protons floating around in solution. Rather, these protons are actually attached to solvent molecules.
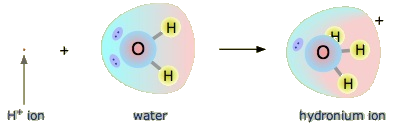
Example 3:
With this in mind, how do we define HCl as an acid in the Brønsted-Lowry sense? Consider what happens when HCl is dissolved in H2O:
\[\ce{HCl(g) + H_2O (ℓ) \rightarrow H_3O^{+}(aq) + Cl^{−}(aq) }\label{Eq2}\]
We can depict this process using Lewis electron dot diagrams:
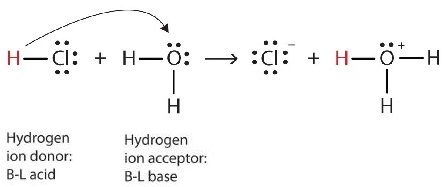
Now we see that a hydrogen ion is transferred from the HCl molecule to the H2O molecule to make chloride ions and hydronium ions. As the hydrogen ion donor, HCl acts as a Brønsted-Lowry acid; as a hydrogen ion acceptor, H2O is a Brønsted-Lowry base.
Amphoteric or Amphiprotic Compounds
Water (H2O) is an interesting compound. Depending on the circumstances, H2O can act as either as a Brønsted-Lowry acid or a Brønsted-Lowry base. In example 1, the water molecule donates a hydrogen ion to the ammonia, it is the Brønsted-Lowry acid. In example 2, the water molecule accepts a hydrogen ion from HCN, it is the Brønsted-Lowry base. In example 3, as a hydrogen ion acceptor, H2O is a Brønsted-Lowry base.
A substance that can either donate or accept a proton, depending on the circumstances, is called amphoteric or amphiprotic.
Water is not the only substance that can react as an acid in some cases or a base in others, but it is certainly the most common example—and the most important one. Other examples are the bicarbonate ion (HCO3-), the hydrogen phosphate (HPO42-) and the dihydrogenphosphate ion (H2PO4-).
A water molecule can act as an acid or a base even in a sample of pure water. About 6 in every 100 million (6 in 108) water molecules undergo the following reaction:
H2O(ℓ)+H2O(ℓ)→H3O+(aq)+OH−(aq)
This process is called the autoionization of water and occurs in every sample of water, whether it is pure or part of a solution. Autoionization occurs to some extent in any amphiprotic liquid. (For comparison, liquid ammonia undergoes autoionization as well, but only about 1 molecule in a million billion (1 in 1015) reacts with another ammonia molecule.)
- A Brønsted-Lowry acid is a proton (hydrogen ion) donor.
- A Brønsted-Lowry base is a proton (hydrogen ion) acceptor.
Example \(\PageIndex{1}\)
Aniline (C6H5NH2) is slightly soluble in water. It has a nitrogen atom that can accept a hydrogen ion from a water molecule, just like the nitrogen atom in ammonia does. Write the chemical equation for this reaction and identify the Brønsted-Lowry acid and base in the reactant side.
Solution
C6H5NH2 and H2O are the reactants. When C6H5NH2 accepts a proton from H2O, it gains an extra H and a positive charge and leaves an OH− ion behind. The reaction is as follows:
\[\ce{C6H5NH2(aq) + H2O(ℓ) <=> C6H5NH3^{+}(aq) + OH^{−}(aq)} \nonumber\]
Because C6H5NH2 accepts a proton, it is the Brønsted-Lowry base. The H2O molecule, because it donates a proton, is the Brønsted-Lowry acid.
Exercise \(\PageIndex{1}\)
Identify the Brønsted-Lowry acid and the Brønsted-Lowry base in the reactant side of this chemical equation.
\[\ce{H2PO4^{-} + H_2O <=> HPO4^{2-} + H3O^{+}}\]
- Answer
- Brønsted-Lowry acid: H2PO4-; Brønsted-Lowry base: H2O
Exercise \(\PageIndex{2}\)
Which of the following compounds is a Bronsted-Lowry base?
- HCl
- HPO42-
- H3PO4
- NH4+
- CH3NH3+
- Answer
-
A Brønsted-Lowry Base is a proton acceptor, which means it will take in an H+. This eliminates \(\ce{HCl}\), \(\ce{H3PO4}\), \(\ce{NH4^{+}}\) and \(\ce{CH_3NH_3^{+}}\) because they are Bronsted-Lowry acids. They all give away protons. In the case of \(\ce{HPO4^{2-}}\), consider the following equation:
\[\ce{HPO4^{2-} (aq) + H2O (l) \rightarrow PO4^{3-} (aq) + H3O^{+}(aq) } \nonumber\]
Here, it is clear that HPO42- is the acid since it donates a proton to water to make H3O+ and PO43-. Now consider the following equation:
\[ \ce{ HPO4^{2-}(aq) + H2O(l) \rightarrow H2PO4^{-} + OH^{-}(aq)} \nonumber\]
In this case, HPO42- is the base since it accepts a proton from water to form H2PO4- and OH-. Thus, HPO42- is an acid and base together, making it amphoteric.
Since HPO42- is the only compound from the options that can act as a base, the answer is (b) HPO42-.
Conjugate Acid-Base Pair
In reality, all acid-base reactions involve the transfer of protons between acids and bases. For example, consider the acid-base reaction that takes place when ammonia is dissolved in water. A water molecule (functioning as an acid) transfers a proton to an ammonia molecule (functioning as a base), yielding the conjugate base of water, \(\ce{OH^-}\), and the conjugate acid of ammonia, \(\ce{NH4+}\):
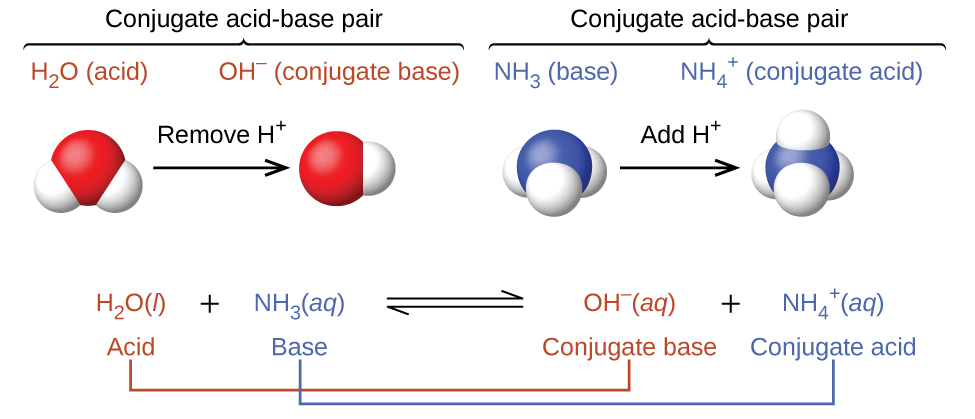
In the reverse reaction, an ammonium ion acts as an acid by donating a proton to a hydroxide ion, and the hydroxide ion acts as a base. The conjugate acid–base pairs for this reaction are \(NH_4^+/NH_3\) and \(H_2O/OH^−\).
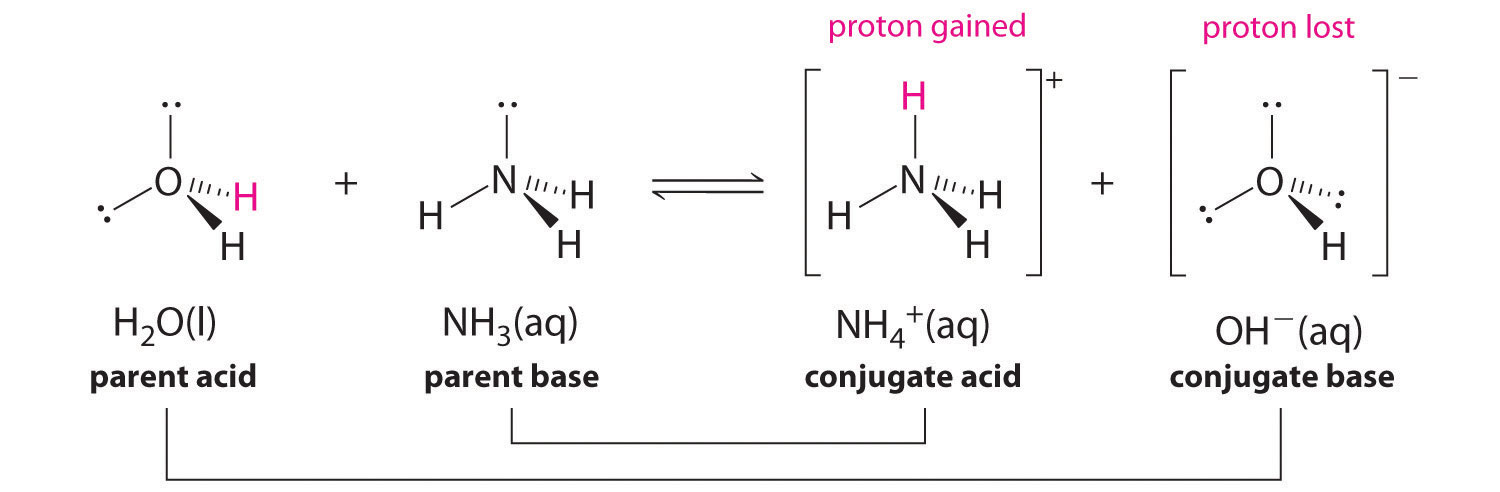
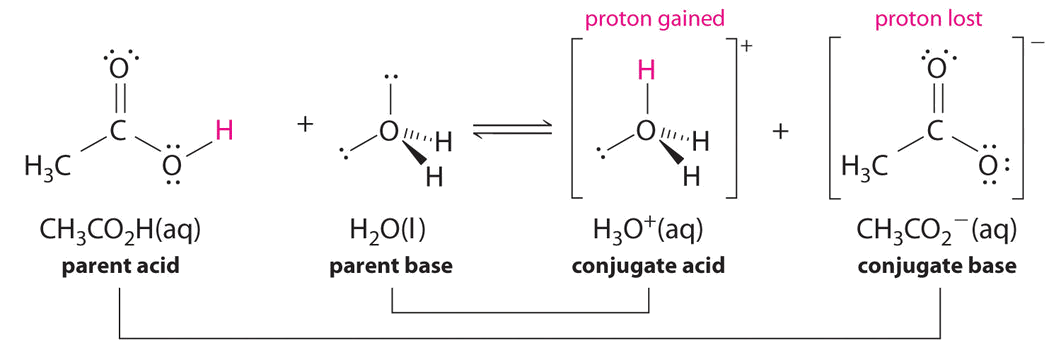
Example \(\PageIndex{2}\)
Identify the conjugate acid-base pairs in this equilibrium.
\[\ce{CH3CO2H + H2O <=> H3O^{+} + CH3CO2^{-}} \nonumber\]
Solution
Similarly, in the reaction of acetic acid with water, acetic acid donates a proton to water, which acts as the base. In the reverse reaction, \(H_3O^+\) is the acid that donates a proton to the acetate ion, which acts as the base.
Once again, we have two conjugate acid-base pairs:
- the parent acid and its conjugate base (\(CH_3CO_2H/CH_3CO_2^−\)) and
- the parent base and its conjugate acid (\(H_3O^+/H_2O\)).
Example \(\PageIndex{3}\)
Identify the conjugate acid-base pairs in this equilibrium.
\[(CH_{3})_{3}N + H_{2}O\rightleftharpoons (CH_{3})_{3}NH^{+} + OH^{-} \nonumber\]
Solution
One pair is H2O and OH−, where H2O has one more H+ and is the conjugate acid, while OH− has one less H+ and is the conjugate base.
The other pair consists of (CH3)3N and (CH3)3NH+, where (CH3)3NH+ is the conjugate acid (it has an additional proton) and (CH3)3N is the conjugate base.
Exercise \(\PageIndex{3}\)
Identify the conjugate acid-base pairs in this equilibrium.
\[\ce{NH2^{-} + H2O\rightleftharpoons NH3 + OH^{-}} \nonumber\]
- Answer
- H2O (acid) and OH− (base); NH2− (base) and NH3 (acid)
Contributions & Attributions
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
Henry Agnew (UC Davis)