8.1: Acid and Base Neutralization
- Page ID
- 432650
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Write and understand acid-base neutralization reactions.
- Write the neutralization reactions between carboxylic acids and bases.
Neutralization Reactions
This is a reaction that happens when an acid, such as \(\ce{HCl}\), is mixed with a base, such as \(\ce{NaOH}\).
\[\ce{HCl (aq) + NaOH (aq) → NaCl (aq) + H_2O (l)}\nonumber \]
When an acid and a base are combined, water and a salt are the products. Salts are ionic compounds containing a positive ion other than H+ and a negative ion other than the hydroxide ion, \(\ce{OH^{-}}\). Double displacement reactions of this type are called neutralization reactions.
When a strong acid and a strong base are combined in the proper amounts, when \([\ce{H^{+}}]\) equals \([\ce{OH^{-}}\)], a neutral solution results in which pH = 7. The acid and base have neutralized each other, and the acidic and basic properties are no longer present.
One of the most concentrated acids in the body is stomach acid, which can be approximated as a 0.05 M hydrochloric acid solution. Special cells in the stomach wall secrete this acid, along with special enzymes, as part of the digestion process. In a laboratory, a 0.05 M solution of hydrochloric acid would dissolve some metals. How does the stomach survive the presence of such a reactive acid?
The stomach has several mechanisms for withstanding this chemical onslaught. First, the lining of the stomach is coated with a thin layer of mucus that contains some bicarbonate ions (HCO3−). These react with the hydrochloric acid to produce water, carbon dioxide, and harmless chloride ions.
HCl(aq) + HCO3-(aq) → HOH(l) + CO2(g) + Cl-(aq)
If any acid penetrates through the mucus, it can attack the surface layer of stomach cells, called the gastric epithelium. Cells in the gastric epithelium are being constantly shed, so damaged cells are quickly removed and replaced with healthy cells.
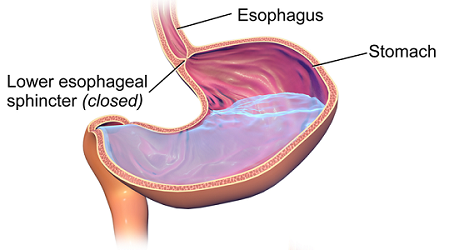
However, if the gastric epithelium is destroyed faster than it can be replaced, the acid may reach the wall of the stomach, resulting in ulcers. If an ulcer grows large enough, it can expose blood vessels in the stomach wall, causing bleeding. In extreme situations, the loss of blood through a severe ulcer can threaten a person’s health.
Ulcers can also result from the presence of a certain bacterium—Helicobacter pylori—in the stomach. The mechanism for this ulcer formation is not the same as that for ulcers caused by stomach acid and is not completely understood. However, there are two main treatments for ulcers: (1) antacids to react chemically with excess hydrochloric acid in the stomach and (2) antibiotics to destroy the H. pylori bacteria in the stomach. Some of the common antacids used to neutralize excess stomach acid are milk of magnesia, Mg(OH)2; Tums, CaCO3; and alka-selzer NaHCO3.
The balanced double displacement reaction between milk of magnesia is shown below. The hydrochloric acid in the stomach is neutralized by the base in milk of magnesia.
Mg(OH)2(s) + 2HCl (aq) → MgCl2(aq) + 2HOH(l)
With tums a neutralization reaction shown in equation 1 between stomach acid and calcium carbonate happens. This double displacement reaction produces carbonic acid, H2CO3 which then decomposes to produce carbon dioxide (CO2) and water (HOH) as shown in equation 2.
CaCO3(s) + 2HCl (aq) → CaCl2(aq) + H2CO3(aq) Equation 1
H2CO3(aq) → CO2(g) + HOH(l) Equation 2
The two equations may be combined and written as one:
CaCO3(s) + 2HCl (aq) → CaCl2(aq) + CO2(g) + HOH(l)
Salt solutions as a result of neutralization reaction do not always have a pH of 7, however. The ions produced when an acid and base combine may react with the water molecules to produce a solution that is slightly acidic or basic. As a general concept, if a strong acid is mixed with a weak base, the resulting solution will be slightly acidic. If a strong base is mixed with a weak acid, the solution will be slightly basic.
Neutralization of Carboxylic Acids
Carboxylic acids are more acidic than other functional groups in organic chemistry. So carboxylic acids will react with bases such as sodium hydroxide (NaOH), sodium carbonate (Na2CO3), and sodium bicarbonate (NaHCO3) to form water and a carboxylic acid salt. Shown below are the neutralization reactions of acetic acid (CH3COOH), a component in vinegar.
CH3COOH + NaOH(aq) → CH3COO−Na+(aq) + H2O
2CH3COOH + Na2CO3(aq) → 2CH3COO−Na+(aq) + H2O + CO2(g)
CH3COOH + NaHCO3(aq) → CH3COO−Na+(aq) + H2O + CO2(g)
In these reactions, the carboxylic acids neutralize basic compounds. With solutions of carbonate and bicarbonate ions, they also form carbon dioxide gas.
Carboxylic acid salts are named in the same manner as inorganic salts: the name of the cation is followed by the name of the organic anion. The name of the anion is obtained by dropping the -ic ending of the acid name and replacing it with the suffix -ate. This rule applies whether we are using common names or International Union of Pure and Applied Chemistry (IUPAC) names:

Example \(\PageIndex{1}\): Propionic Acid + Calcium Hydroxide
Calcium propionate is used to inhibit the growth of molds in foods, tobacco, and some medicines. Write a balanced chemical equation for the reaction of aqueous propionic acid (CH3CH2CO2H) with aqueous calcium hydroxide [Ca(OH)2].
Solution
| Steps | Reaction |
|---|---|
|
Write the unbalanced equation. This is a double displacement reaction, so the cations and anions swap to create the water and the salt. |
CH3CH2CO2H(aq) + Ca(OH)2(aq)→(CH3CH2CO2)2Ca(aq) + H2O(l) |
|
Balance the equation. Because there are two OH− ions in the formula for Ca(OH)2, we need two moles of propionic acid, CH3CH2CO2H, to provide H+ ions. |
2CH3CH2CO2H(aq) + Ca(OH)2(aq)→(CH3CH2CO2)2Ca(aq) +2H2O(l) |
Exercise \(\PageIndex{1}\)
Write a balanced chemical equation for the reaction of solid barium hydroxide with dilute acetic acid.
- Answer
-
\[\ce{Ba(OH)2(s) + 2CH3CO2H (aq)→Ba(CH3CO2)2 (aq) + 2H2O(l)} \nonumber\nonumber \]
Contributions & Attributions
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
- Deboleena Roy, Ph.D. Dept. of Science and Engineering @ American River College.
Peggy Lawson (Oxbow Prairie Heights School). Funded by Saskatchewan Educational Technology Consortium.
Henry Agnew (UC Davis)


