7.11: End of Chapter Problems
- Page ID
- 440200
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Classification of Monosaccharides
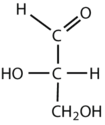
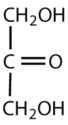
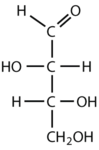
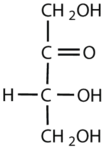
What is the classification of the following monosaccharides in terms of the # of carbon atoms and functional groups.
Common Monosaccharides
1. Name two common pentoses and draw their structure in the Fischer Projection formula.
2. Name three common hexoses and draw their structure in the Fischer Projection formula.
3. How does an alpha anomer differ from a beta anomer?
4. How does a pyranose differ from a furanose?
5. Draw each molecule.
a. α-glucopyranose
b. α-galactopyranose
c. β-fructofuranose
d. β-ribofuranose
e. β-2-deoxyribofuranose
Oligosaccharides
1. For the trisaccharides, identify the glycosidic linkages.
a. 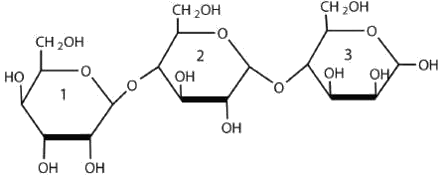
b. 
2. Sucralose is an artificial sweetener that is 600 times sweeter than sucrose and does have a stricture that resembles a disaccharide. This sweetener does not add calories to your diet. It is stable at high temperature can be widely used for cooking and baking

a. Identify the glycosidic linkage.
b. What are two monosaccharides derivatives this disaccharide is made of?
3. Stachyose is an indigestible oligosaccharide present in peas and beans. This oligosaccharide contain galactopyranose residues involved in  -(1
-(1 6) glycosidic bond. Humans do not produce the
6) glycosidic bond. Humans do not produce the  -galactosidase enzyme necessary to hydrolyze this bond. So when foods containing these carbohydrates are eaten, intestinal bacteria break this oligosaccharide down and produce gases. Products like "beano" contain an enzyme that catalyse the hydrolysis of the
-galactosidase enzyme necessary to hydrolyze this bond. So when foods containing these carbohydrates are eaten, intestinal bacteria break this oligosaccharide down and produce gases. Products like "beano" contain an enzyme that catalyse the hydrolysis of the  -(1-6) glycosidic bond and may be taken with a meal to prevent discomfort.
-(1-6) glycosidic bond and may be taken with a meal to prevent discomfort.

a. Identify the type of glycosidic bonds in stachyose.
b. Identify the monosaccharides in stachyose.
c. Label the rings as pyranose or furanose.
Polysaccharides
1. Compare the structures of amylose and amylopectin. How are they similar and how are they different?
2. Compare the structures of amylose and cellulose. How are they similar and how are they different?
3. Compare the structures of glycogen and amylopectin. How are they similar and how are they different?
4. Why can horses use cellulose as a food source?
5. Why can humans use starch as a food source, but not cellulose?
Digestion
1. Where in the digestive system does the digestion of each of the following take place?
a. starch b. maltose c. sucrose d. lactose
2. What monosaccharides are formed when each of the following undergoes digestion and are broken down to its building blocks?
a. starch b. maltose c. sucrose d. lactose
3. Name the glycosidic bond hydrolyzed when each of the following undergoes digestion?
a. starch b. maltose c. sucrose d. lactose
4. Name the enzyme that catalyzes a reaction involved in the digestion of the following?
a. starch b. maltose c. sucrose d. lactose
5. Name three common disaccharides in our diet and name the monosaccharides formed when they are hydrolyzed in the small intestine?
6. Only a small fraction of the starch in food is digested once it enters the small intestine. Why?
7. What are the two glycosidic bonds that must be hydrolyzed to digest amylopectin to individual glucose molecules? Does the same enzyme hydrolyze both types of glycosidic bonds?
Glucose Metabolism
1. What is the role of glycolysis?
2. What is the net change in ATP and NADH from the passage of one glucose molecule through glycolysis?
3. In what part of the cell does glycolysis take place?
4. Glycolysis is described as a process in which energy is invested "up front" in exchange for greater return of energy later on. Which steps in glycolysis involve investment of energy?
5. What is the fate of the pyruvate ion under anaerobic conditions in humans? What is the purpose of this conversion?
6. What is the fate of the pyruvate ion during fermentation in yeast cells? What is the purpose of this conversion?
7. What is the fate of the pyruvate ion during aerobic conditions? What is the purpose of this conversion? What part of the cell does this conversion happen?
8. What is gluconeogenesis? What is the role of this pathway?
9. Which compound is common to the gluconeogenesis pathway and the citric acid cycle?
10. What are the three steps in glycolysis that cannot be reversed in gluconeogenesis?
11. What are ketone bodies? How are they produced?
12. A "low carb" diet craze swept the United States in 2004.
a. Explain how this diet can lead to overproduction of ketone bodies.
b. One effect of this diet was that people had "sickeningly sweet" bad breath (keto breath). What molecule is responsible for the bad breath?