5.7: Oxidations and Reductions
- Page ID
- 429091
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Define oxidation and reductions
- Identify a chemical reaction as an oxidation-reduction reaction based on their definition.
Oxidation-reduction reactions are of central importance in organic chemistry and biochemistry. The burning of fuels that provides the energy to maintain our civilization and the metabolism of foods that furnish the energy that keeps us alive both involve oxidation-reduction reactions.
Oxidation
The origin of the term oxidation is “adding oxygen,” so when oxygen is added to a molecule, the molecule is being oxidized. For example, the aldehyde molecule takes on an oxygen atom (marked purple) to become a carboxylic acid. Thus, the aldehyde molecule is being oxidized.

Similarly, oxidation can be defined in terms of the loss of hydrogen atoms. If a molecule loses hydrogen atoms, the molecule is being oxidized. For example, in the conversion of the 1o alcohol to aldehyde, the alcohol loses two hydrogen atoms (marked purple).

Oxidation may also be defined as the loss of electrons by an atom. An example, is the single displacement reaction of sodium metal with water
2Na(s) + 2HOH(l) → H2(g) + 2NaOH(aq)
In this reaction sodium metal Na(s) loses and an electron to become sodium ion (Na+) in the ionic compound NaOH. So, sodium atom is oxidized.
Na → Na+ + e-
Applications of Oxidation in Biochemistry
Oxidation reactions are found in many metabolic process and happen within the living cells. Here are a couple of examples from the citric acid cycle.
a. The citric acid cycle is a series of reactions important to metabolism. One of the steps (step 8) in the cycle is the conversion of the malate (2o-alcohol) to oxaloacetate (ketone). The alcohol loses two hydrogen atoms (marked purple). Malate is oxidized because it has undergone oxidation. NAD+ is the oxidizing agent. NAD+ is reduced to NADH and H+ ion.

b. Another step in the cycle (step 6) involves the conversion of the succinate to fumarate, succinate loses two hydrogen atoms (marked purple) to an alkene fumarate. FAD is the oxidizing agent. FAD is reduced to FADH2.

NAD+ and FAD are biological oxidizing agents called oxidized coenzymes.
Reduction
The reverse is true for reduction: if a molecule loses oxygen atoms, the molecule is being reduced.
If a molecule adds hydrogen atoms, it is being reduced.
For example, in the conversion of alkene into alkane, hydrogen atoms are added to alkene (in purple), so the alkene is being reduced.

In the conversion of aldehyde into 1o alcohol below, hydrogen atoms are added (in purple) to the aldehyde, so the aldehyde is being reduced.

In the conversion of ketone into 2o alcohol below, hydrogen atoms are added (in purple) to the ketone, so the ketone is being reduced.
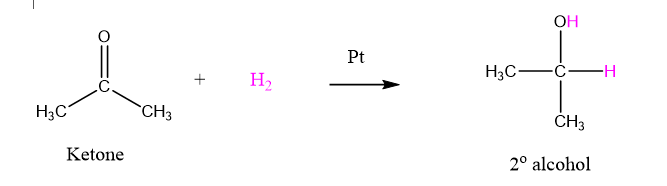
Reduction may also be defined as the gain of electrons by an atom.
Applications of Reduction in Biochemistry
Reduction reactions are found in many metabolic process and happen within the living cells. Here are a couple of examples from glucose metabolism.
a. In the conversion of acetaldehyde to ethanol, the aldehyde gains two hydrogen atoms (marked purple). Acetaldehyde is reduced because it has undergone reduction. NADH is the reducing agent. NADH is reduced to NAD+. This step is important in yeast during the fermentation of glucose from fruits and grains to produce ethanol (drinking alcohol).

b. In the conversion of pyruvate to lactate, the ketone functional group gains two hydrogen atoms (marked purple). Pyruvate is reduced because it has undergone reduction. NADH is the reducing agent. NADH is reduced to NAD+. Lactate is produced during anaerobic (O2 deficient) conditions of the cell such as when a person is exercising vigorously.

NADH and FADH2 are biological reducing agents called reduced coenzymes.
Other Applications of Reduction
a. Artificial Sweeteners:
Alcohol sugars such as sorbitol and xylitol are made by the reduction reaction of aldehydes.


Sorbitol is used to sweeten food for diabetics. The aldehyde functional group in glucose gains two hydrogen atoms from H2 to form sorbitol (a 1o-alcohol).
Xylitol is used for sugarless gums. The aldehyde functional group in xylose gains two hydrogen atoms from H2 to form xylitol (a 1o-alcohol).
b. Partially Hydrogenated Vegetable Oils
Partial hydrogenation of a triglyceride converts a vegetable oil into a semisolid product called margarine which has a good consistency for cooking but does not contain cholesterol found in animal fats. The vegetable oil shown below has 4 alkene functional groups. Two of the alkenes (shown in purple) undergo hydrogenation to alkane.

This page was constructed by Deboleena Roy (American River College) from the following sources:
5.6: Redox Reactions in Organic Chemistry and Biochemistry - Chemistry LibreTexts


