3.4: Polyatomic Ions and Formulae for Ionic Compounds
- Page ID
- 430553
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Recognize polyatomic ions in chemical formulas.
- Write the chemical formula for an ionic compound and name them.
We have already encountered some chemical formulas for ionic compounds from monoatomic ions. A chemical formula is a concise list of the elements in a compound and the ratios of these elements. To better understand what a chemical formula means, we must consider how an ionic compound is constructed from its ions.
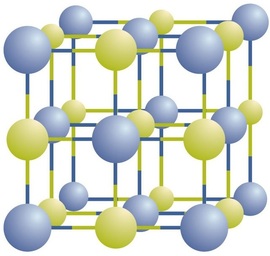
Ionic compounds exist as alternating positive and negative ions in regular, three-dimensional arrays called crystals (Figure \(\PageIndex{1}\)). As you can see, there are no individual \(\ce{NaCl}\) “particles” in the array; instead, there is a continuous lattice of alternating sodium and chloride ions. However, we can use the ratio of sodium ions to chloride ions, expressed in the lowest possible whole numbers, as a way of describing the compound. In the case of sodium chloride, the ratio of sodium ions to chloride ions, expressed in lowest whole numbers, is 1:1, so we use \(\ce{NaCl}\) (one \(\ce{Na}\) symbol and one \(\ce{Cl}\) symbol) to represent the compound. Thus, \(\ce{NaCl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions in the compound. A macroscopic sample is composed of myriads of NaCl pairs; each individual pair called a formula unit. Although it is convenient to think that \(\ce{NaCl}\) crystals are composed of individual \(\ce{NaCl}\) units, Figure \(\PageIndex{1}\) shows that no single ion is exclusively associated with any other single ion. Each ion is surrounded by ions of opposite charge.
Polyatomic Ions
Some ions consist of groups of atoms covalently bonded together and have an overall electric charge. Because these ions contain more than one atom, they are called polyatomic ions. The Lewis structures, names and formulas of some polyatomic ions are found in Figure 3.4.1.
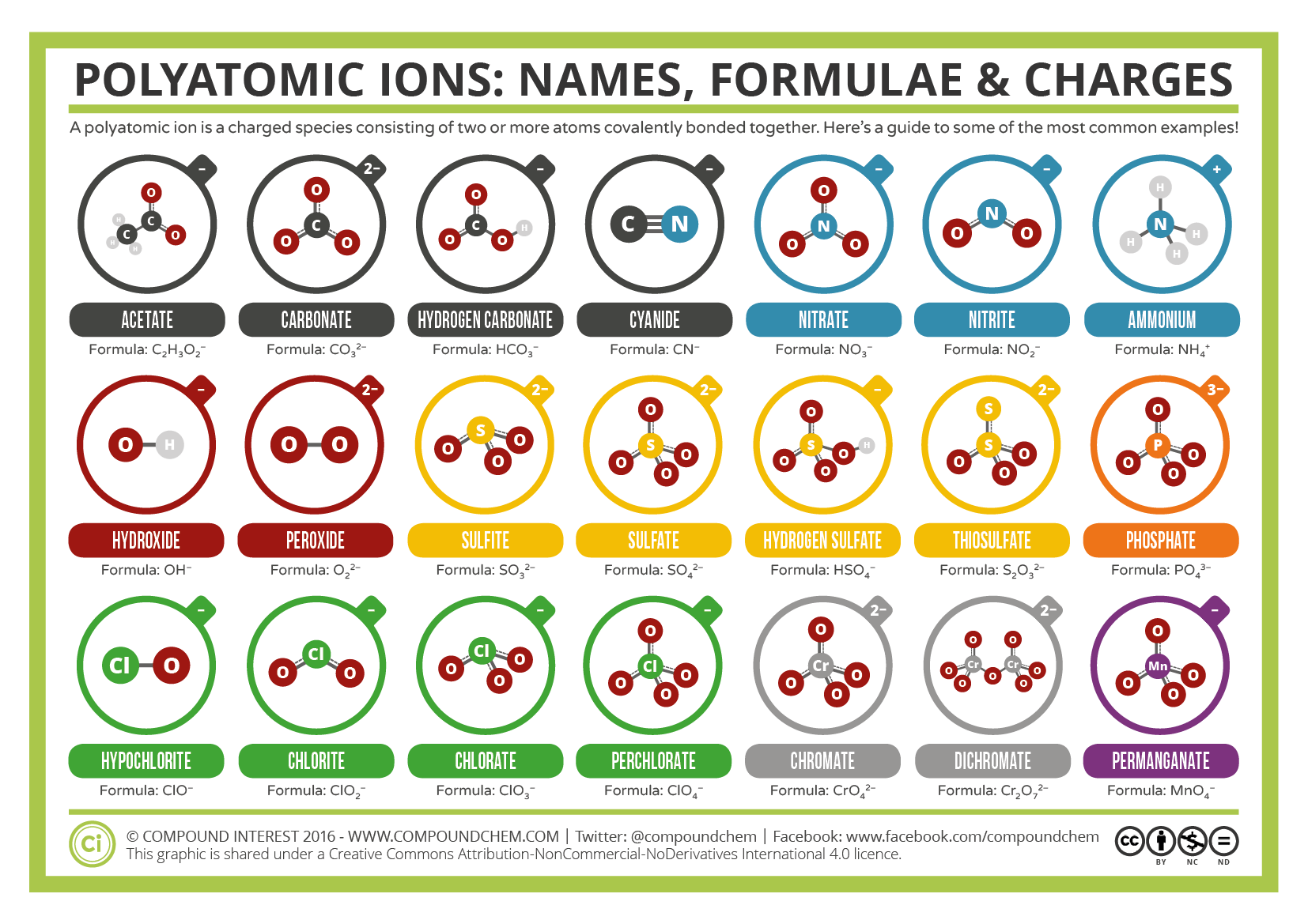
Figure \(\PageIndex{1}\): Names, formula, and charges of polyatomic ions
Polyatomic ions have defined formulas, names, and charges that cannot be modified in any way. Table \(\PageIndex{1}\) lists the ion names and ion formulas of the most common polyatomic ions. For example, \(\ce{NO3^{−}}\) is the nitrate ion; it has one nitrogen atom and three oxygen atoms and an overall 1− charge.
| Ion Name | Ion Formula |
|---|---|
| ammonium ion | NH4+1 |
| hydronium ion | H3O+1 |
| hydroxide ion | OH−1 |
| cyanide ion | CN−1 |
| carbonate ion | CO3−2 |
| bicarbonate or hydrogen carbonate | HCO3− |
| acetate ion | C2H3O2−1 or CH3CO2−1 |
| nitrate ion | NO3−1 |
| nitrite ion | NO2−1 |
| sulfate ion | SO4−2 |
| sulfite ion | SO3−2 |
| phosphate ion | PO4−3 |
| phosphite ion | PO3−3 |
Note that there are two polyatomic ions in this table, the ammonium ion and hydronium ion that are cations. The ammonium ion contains one nitrogen and four hydrogen's that collectively bear a +1 charge. The hydronium ion contains one oxygen and three hydrogen's that collectively bear a +1 charge.
The remaining polyatomic ions are all negatively-charged and, therefore, are classified as anions. However, only two of these, the hydroxide ion and the cyanide ion, are named using the "-ide" suffix that is typically indicative of negatively-charged particles.
The remaining polyatomic anions, which all contain oxygen, in combination with another non-metal, exist as part of a series in which the number of oxygen's within the polyatomic unit can vary. No two chemical formulas should share a common chemical name. A single suffix, "-ide," is insufficient for distinguishing the names of the anions in a related polyatomic series. Therefore, "-ate" and "-ite" suffixes are employed, in order to denote that the corresponding polyatomic ions are part of a series. Additionally, these suffixes also indicate the relative number of oxygens that are contained within the polyatomic ions. Note that all of the polyatomic ions whose names end in "-ate" contain one more oxygen than those polyatomic anions whose names end in "-ite." Unfortunately, much like the common system for naming transition metals, these suffixes only indicate the relative number of oxygens that are contained within the polyatomic ions. For example, the nitrate ion, which is symbolized as NO3−1, has one more oxygen than the nitrite ion, which is symbolized as NO2−1. However, the sulfate ion is symbolized as SO4−2. While both the nitrate ion and the sulfate ion share an "-ate" suffix, the former contains three oxygens, but the latter contains four. Additionally, both the nitrate ion and the sulfite ion contain three oxygens, but these polyatomic ions do not share a common suffix. Unfortunately, the relative nature of these suffixes mandates that the ion formula/ion name combinations of the polyatomic ions must simply be memorized.
Most of the polyatomic ions in table 3.4.1 are found in significant amounts in all living things and required for survival. For example, the hydrogen carbonate ion (HCO3-) is involved in the transport of carbon dioxide (CO2) from the tissues to the lungs.
Naming Compounds
Now that we know how to name ions, we are ready to name ionic compounds. We do so by placing the name of the cation first, followed by the name of the anion, and dropping the word ion from both parts. For example, what is the name of the compound whose formula is \(\ce{Ba(NO3)2}\)?
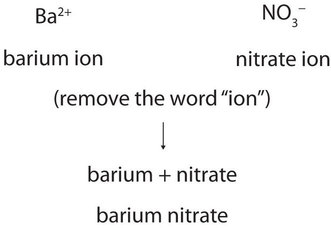
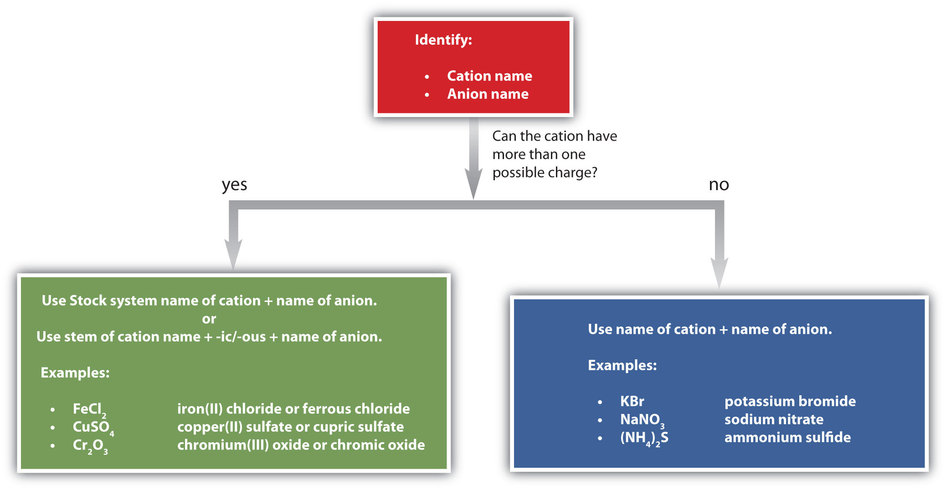
The compound’s name does not indicate that there are two nitrate ions for every barium ion. You must determine the relative numbers of ions by balancing the positive and negative charges. If you are given a formula for an ionic compound whose cation can have more than one possible charge, you must first determine the charge on the cation before identifying its correct name. For example, consider \(\ce{FeCl2}\) and \(\ce{FeCl3}\). In the first compound, the iron ion has a 2+ charge because there are two \(\ce{Cl^{−}}\) ions in the formula (1− charge on each chloride ion). In the second compound, the iron ion has a 3+ charge, as indicated by the three \(\ce{Cl^{−}}\) ions in the formula. These are two different compounds that need two different names. By the Stock system, the names are iron(II) chloride and iron(III) chloride. If we were to use the stems and suffixes of the common system, the names would be ferrous chloride and ferric chloride, respectively.
Formulae for Ionic Compounds from Polyatomic Ions
The rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing monatomic (single-atom) ions: the positive and negative charges must balance. If more than one of a particular polyatomic ion is needed to balance the charge, the entire formula for the polyatomic ion must be enclosed in parentheses, and the numerical subscript is placed outside the parentheses. This is to show that the subscript applies to the entire polyatomic ion. Two examples are shown below:

Write the chemical formula for an ionic compound composed of each pair of ions.
- the potassium ion and the sulfate ion
- the calcium ion and the nitrate ion
Solution
- Potassium ions have a charge of 1+, while sulfate ions have a charge of 2−. We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{K_2SO_4}\).
- Calcium ions have a charge of 2+, while nitrate ions have a charge of 1−. We will need two nitrate ions to balance the charge on each calcium ion. The formula for nitrate must be enclosed in parentheses. Thus, we write \(\ce{Ca(NO3)2}\) as the formula for this ionic compound.
Write the chemical formula for an ionic compound composed of each pair of ions.
- the magnesium ion and the carbonate ion
- the aluminum ion and the acetate ion
- Answer a:
-
Mg2+ and CO32- = MgCO3
- Answer b:
-
Al3+ and C2H3O2- = Al(C2H3O2)3
Recognizing Ionic Compounds
There are two ways to recognize ionic compounds. First, compounds between metal and nonmetal elements are usually ionic. For example, CaBr2 contains a metallic element (calcium, a group 2A metal) and a nonmetallic element (bromine, a group 7A nonmetal). Therefore, it is an ionic compound. In contrast, the compound NO2 contains two elements that are both nonmetals (nitrogen, from group 5A, and oxygen, from group 6A). It is not an ionic compound; it belongs to the category of covalent compounds also know as molecular compounds. Also note that this combination of nitrogen and oxygen has no electric charge specified, so it is not the nitrite ion.
Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. For example, if you see the formula \(\ce{Ba(NO3)2}\), you may recognize the “NO3” part as the nitrate ion, \(\rm{NO_3^−}\). (Remember that the convention for writing formulas for ionic compounds is not to include the ionic charge.) This is a clue that the other part of the formula, \(\ce{Ba}\), is actually the \(\ce{Ba^{2+}}\) ion, with the 2+ charge balancing the overall 2− charge from the two nitrate ions. Thus, this compound is also ionic.
If the compound is not ionic then it is a molecular compound.
Identify each compound as ionic or molecular (not ionic).
- \(\ce{Na2O}\)
- \(\ce{PCl3}\)
- \(\ce{NH4Cl}\)
- \(\ce{OF2}\)
Solution
- Sodium is a metal, and oxygen is a nonmetal; therefore, \(\ce{Na2O}\) is expected to be ionic.
- Both phosphorus and chlorine are nonmetals. Therefore, \(\ce{PCl3}\) is a molecular compound.
- The \(\ce{NH4}\) in the formula represents the ammonium ion, \(\ce{NH4^{+}}\), which indicates that this compound is ionic.
- Both oxygen and fluorine are nonmetals. Therefore, \(\ce{OF2}\) is not ionic.
Identify each compound as ionic or molecular (not ionic).
- \(\ce{N2O}\)
- \(\ce{FeCl3}\)
- \(\ce{(NH4)3PO4}\)
- \(\ce{SOCl2}\)
- Answer a:
-
molecular
- Answer b:
-
ionic
- Answer c:
-
ionic
- Answer d:
-
molecular
Science has long recognized that blood and seawater have similar compositions. After all, both liquids have ionic compounds dissolved in them. The similarity may be more than mere coincidence; many scientists think that the first forms of life on Earth arose in the oceans. A closer look, however, shows that blood and seawater are quite different. A 0.9% solution of sodium chloride approximates the salt concentration found in blood. In contrast, seawater is principally a 3% sodium chloride solution, over three times the concentration in blood. Here is a comparison of the amounts of ions in blood and seawater:
| Ion | Percent in Seawater | Percent in Blood |
|---|---|---|
| Na+ | 2.36 | 0.322 |
| Cl− | 1.94 | 0.366 |
| Mg2+ | 0.13 | 0.002 |
| SO42− | 0.09 | — |
| K+ | 0.04 | 0.016 |
| Ca2+ | 0.04 | 0.0096 |
| HCO3− | 0.002 | 0.165 |
| HPO42−, H2PO4− | — | 0.01 |
Most ions are more abundant in seawater than they are in blood, with some important exceptions. There are far more hydrogen carbonate ions (\(\ce{HCO3^{−}}\)) in blood than in seawater. This difference is significant because the hydrogen carbonate ion and some related ions have a crucial role in controlling the acid-base properties of blood. The amount of hydrogen phosphate ions—\(\ce{HPO4^{2−}}\) and \(\ce{H2PO4^{−}}\)—in seawater is very low, but they are present in higher amounts in blood, where they also affect acid-base properties. Another notable difference is that blood does not have significant amounts of the sulfate ion (\(\ce{SO4^{2−}}\)), but this ion is present in seawater.
A synopsis of how to name simple ionic compounds is shown in figure 3.4.2.
Key Takeaways
- Proper chemical formulas for ionic compounds balance the total positive charge with the total negative charge.
- Groups of atoms with an overall charge, called polyatomic ions, also exist.
EXERCISES
- What information is contained in the formula of an ionic compound?
- Why do the chemical formulas for some ionic compounds contain subscripts, while others do not?
3. Write the chemical formula for the ionic compound formed by each pair of ions.
- Mg2+ and I−
- Na+ and O2−
4. Write the chemical formula for the ionic compound formed by each pair of ions.
- Na+ and Br−
- Mg2+ and Br−
- Mg2+ and S2−
5. Write the chemical formula for the ionic compound formed by each pair of ions.
- K+ and Cl−
- Mg2+ and Cl−
- Mg2+ and Se2−
6. Write the chemical formula for the ionic compound formed by each pair of ions.
- Na+ and N3−
- Mg2+ and N3−
- Al3+ and S2−
7. Write the chemical formula for the ionic compound formed by each pair of ions.
- Li+ and N3−
- Mg2+ and P3−
- Li+ and P3−
8. Write the chemical formula for the ionic compound formed by each pair of ions.
- Fe3+ and Br−
- Fe2+ and Br−
- Au3+ and S2−
- Au+ and S2−
9. Write the chemical formula for the ionic compound formed by each pair of ions.
- Cr3+ and O2−
- Cr2+ and O2−
- Pb2+ and Cl−
- Pb4+ and Cl−
10. Write the chemical formula for the ionic compound formed by each pair of ions.
- Cr3+ and NO3−
- Fe2+ and PO43−
- Ca2+ and CrO42−
- Al3+ and OH−
11. Write the chemical formula for the ionic compound formed by each pair of ions.
- NH4+ and NO3−
- H+ and Cr2O72−
- Cu+ and CO32−
- Na+ and HCO3−
12. For each pair of elements, determine the charge for their ions and write the proper formula for the resulting ionic compound between them.
- Ba and S
- Cs and I
13. For each pair of elements, determine the charge for their ions and write the proper formula for the resulting ionic compound between them.
- K and S
- Sc and Br
14. Which compounds would you predict to be ionic?
- Li2O
- (NH4)2O
- CO2
- FeSO3
- C6H6
- C2H6O
15. Which compounds would you predict to be ionic?
- Ba(OH)2
- CH2O
- NH2CONH2
- (NH4)2CrO4
- C8H18
- NH3
12. Name the ionic compound formed by each pair of ions. Use both the Stock and common systems, where appropriate.
- Cr3+ and NO3−
- Fe2+ and PO43−
- Ca2+ and CrO42−
- Al3+ and OH−
13. Name the ionic compound formed by each pair of ions. Use both the Stock and common systems, where appropriate.
- NH4+ and NO3−
- K+ and Cr2O72−
- Cu+ and CO32−
- Na+ and HCO3−
14. Give two names for each compound.
- Al(HSO4)3
- Mg(HSO4)2
15. Give two names for each compound.
- Co(HCO3)2
- LiHCO3
11.
- chromium(III) oxide or chromic oxide
- chromium(II) oxide or chromous oxide
- lead(II) chloride or plumbous chloride
- lead(IV) chloride or plumbic chloride
12.
- chromium(III) nitrate or chromic nitrate
- iron(II) phosphate or ferrous phosphate
- calcium chromate
- aluminum hydroxide
13.
- ammonium nitrate
- potassium dichromate
- copper(I) carbonate or cuprous carbonate
- sodium hydrogen carbonate or sodium bicarbonate
14.
- aluminum hydrogen sulfate or aluminum bisulfate
- magnesium hydrogen sulfate or magnesium bisulfate
15.
- cobalt hydrogen carbonate or cobalt bicarbonate
- lithium hydrogen carbonate or lithium bicarbonate
Answers
1. the ratio of each kind of ion in the compound
2. Sometimes more than one ion is needed to balance the charge on the other ion in an ionic compound.
3.
- MgI2
- Na2O
4.
- NaBr
- MgBr2
- MgS
5.
- KCl
- MgCl2
- MgSe
6.
- Na3N
- Mg3N2
- Al2S3
7.
- Li3N
- Mg3P2
- Li3P
8.
- FeBr3
- FeBr2
- Au2S3
- Au2S
9.
- Cr2O3
- CrO
- PbCl2
- PbCl4
10.
- Cr(NO3)3
- Fe3(PO4)2
- CaCrO4
- Al(OH)3
11.
- NH4NO3
- H2Cr2O7
- Cu2CO3
- NaHCO3
12.
- Ba2+, S2−, BaS
- Cs+, I−, CsI
13.
- K+, S2−, K2S
- Sc3+, Br−, ScBr3
14.
- ionic
- ionic
- not ionic
- ionic
- not ionic
- not ionic
15.
- ionic
- not ionic
- not ionic
- ionic
- not ionic
- not ionic


