3.2: Ions-Main Group Elements
- Page ID
- 430552
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Use electron dot diagrams to illustrate ion formation.
There is an electrostatic attraction between opposite charged ions such as sodium cation and chloride ion. The resulting combination is the compound sodium chloride. The number of electrons lost by the sodium atom (one) equals the number of electrons gained by the chlorine atom (one), so the compound is electrically neutral. In macroscopic samples of sodium chloride, there are billions and billions of sodium and chloride ions, although there is always the same number of cations and anions.
Ions of the Main Group Elements
Elements that belong to the same main group (vertical column) on the periodic table form ions with the same charge because they have the same number of valence electrons. Thus, the periodic table becomes a tool for remembering the charges on many ions.
For example, all ions made from alkali metals, the first column on the periodic table, have a 1+ charge.
Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge.
On the other side of the periodic table, the next-to-last column, the halogens, form ions having a 1− charge.
The figure 3.2.1 below shows how the charge on many ions can be predicted by the location of an element on the periodic table.
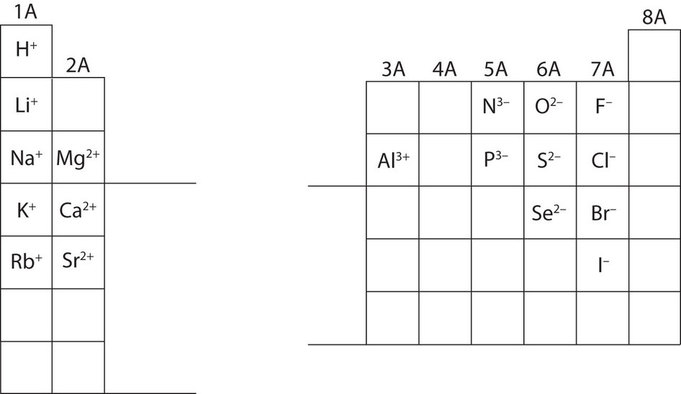
Which of these ions is not likely to form?
- Mg+
- K+
Solution
(a) Mg is in Group 2A and has two valence electrons. It achieves octet by losing two electrons to form Mg2+ cation. Losing only one electron to form Mg+ does not make an octet, hence, Mg+ is not likely to form.
Exercise \(\PageIndex{2}\)
Which of these ions is not likely to form?
- S3−
- N3−
- Answer
-
(a) S is in Group 6A and has six valence electrons. It achieves octet by gaining two electrons to form S2− anion. Gaining three electrons to form S3−does not make it octet, hence, S3− is not likely to form.
Cations of the Main Group Elements
The table 3.2.1 below lists some common ions of the main group metals. Monoatomic cations formed from the main group are given the same name as the original element. So Na+ is sodium ion and Ca2+ is calcium ion. While most main group element form only one cation, elements of the group 4A form two different cations by losing 2 or 4 valence electrons. While naming these cations of lead and tin, a Roman numeral (in parentheses) is used to indicate the charge. Sn2+ is Tin(II) ion and Sn4+ is Tin(IV) ion.
Table \(\PageIndex{1}\): Some Monatomic Cations of the Main Group Elements
|
Group # |
Cation formula |
Name of cation |
|
1A |
Li+ |
Lithium ion |
|
1A |
Na+ |
Sodium ion |
|
1A |
K+ |
Potassium ion |
|
2A |
Mg+2 |
Magnesium ion |
|
2A |
Ca+2 |
Calcium ion |
|
2A |
Sr+2 |
Strontium ion |
|
2A |
Ba+2 |
Barium ion |
|
3A |
Al+3 |
Aluminum ion |
|
4A |
Sn2+ |
tin(II) ion or stannous ion |
|
4A |
Sn4+ |
tin(IV) ion or stannic ion |
|
4A |
Pb2+ |
lead(II) ion or plumbous ion |
|
4A |
Pb4+ |
lead(IV) ion or plumbic ion |
Anions of the Main Group Elements
The table 3.2.2 below lists some common anions of the main group nonmetals.The name of a monatomic anion consists of the stem of the element name, the suffix -ide, and then the word ion. Thus, Cl− is “chlor-” + “-ide ion,” or the chloride ion. Similarly, O2− is the oxide ion, N3− is the nitride ion, and so forth.
Table \(\PageIndex{2}\): Some Monatomic Anions of the Main Group Elements
|
Group # |
Anion formula |
Name |
|
7A |
F- |
fluoride ion |
|
7A |
Cl- |
chloride ion |
|
7A |
Br- |
bromide ion |
|
7A |
I- |
iodide ion |
|
6A |
O2- |
oxide ion |
|
6A |
S2- |
sulfide ion |
|
5A |
N3- |
nitride ion |
|
5A |
P3- |
phosphide ion |
The enamel of your teeth is composed of an ionic compound called calcium hydroxyapatite, Ca5(OH)(PO4)3. Acids produced by bacteria present in dental plaque breaks down the enamel. As a result tooth decay happens. Fluoride ion (F-) is used to prevent tooth decay since the anion converts hydroxy apatite to fluoroapatite, Ca5F(PO4)3. Fluoroapatite is more durable and hence is not broken down easily by acids.
Electron Dot Diagrams
Chemists use simple diagrams to show an atom’s valence electrons and how they transfer. They show only valence electrons, each dot represents a valence electron. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. For example, the representation for sodium is as follows:

and the representation for chlorine is as follows:

For the above diagrams, it does not matter what sides the dots are placed on in Lewis diagrams as long as each side has a maximum of two dots.
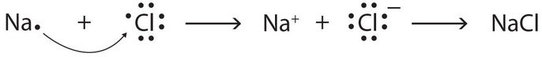
Figure \(\PageIndex{2}\): The transfer of electrons can be illustrated with Lewis diagrams.
In representing the final formula, the dots are omitted.
Starting with lithium and bromine atoms, use Lewis diagrams to show the formation of the ionic compound LiBr.
Solution
From the periodic table, we see that lithium is in the same column as sodium, so it will have the same valence shell electron configuration. That means that the neutral lithium atom will have the same Lewis diagram that the sodium atom has. Similarly, bromine is in the same column as chlorine, so it will have the same Lewis diagram that chlorine has. Therefore,
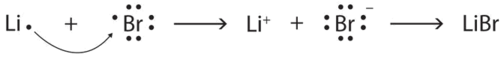
Starting with magnesium and oxygen atoms, use Lewis diagrams to show the formation of the ionic compound MgO.
- Answer
-
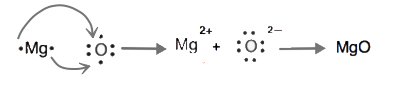
Some ionic compounds have different numbers of cations and anions. In those cases, electron transfer occurs between more than one atom. For example, here is the formation of MgBr2:

Notice that in this example there are two bromide ions (1– charge) needed for every one magnesium ion (2+ charge) in order for the overall charge of the compound to equal zero. This is called charge balance. The number of each type of ion is indicated in the formula by the subscript.
Most of the elements that make ionic compounds form an ion that has a characteristic charge. For example, sodium makes ionic compounds in which the sodium ion always has a 1+ charge. Chlorine makes ionic compounds in which the chloride ion always has a 1− charge. Some elements, especially transition metals, can form ions of multiple charges.
Key Takeaways
- Ions can be positively charged or negatively charged.
- A Lewis diagram is used to show how electrons are transferred to make ions and ionic compounds.
Exercises
- What are the two types of ions?
- Use Lewis diagrams to illustrate the formation of an ionic compound from a potassium atom and an iodine atom.
3. When the following atoms become ions, what charges do they acquire?
- Li
- S
- Ca
- F
4. Identify each as a cation, an anion, or neither.
- H+
- Cl−
- O2
- Ba2+
- CH4
- CS2
5. Identify each as a cation, an anion, or neither.
- NH3
- Br−
- H−
- Hg2+
- CCl4
- SO3
6. Using Lewis diagrams, show the electron transfer for the formation of LiF.
7. Using Lewis diagrams, show the electron transfer for the formation of MgO.
8. Using Lewis diagrams, show the electron transfer for the formation of Li2O.
9. Using Lewis diagrams, show the electron transfer for the formation of CaF2.
10. What characteristic charge do atoms in the first column of the periodic table have when they become ions?
11. What characteristic charge do atoms in the second column of the periodic table have when they become ions?
12. What characteristic charge do atoms in the third-to-last column of the periodic table have when they become ions?
13. What characteristic charge do atoms in the next-to-last column of the periodic table have when they become ions?
Answers
- Cations (positive charged) and anions (negative charged).
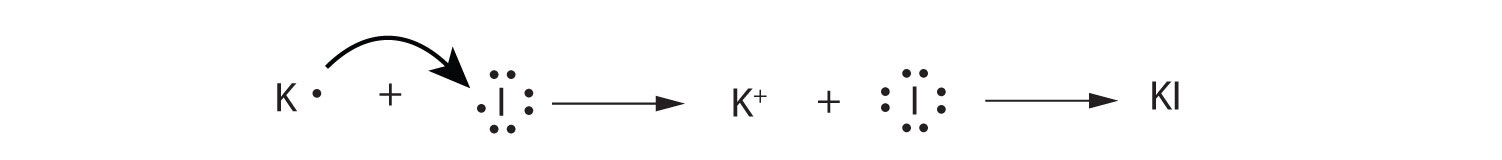
-
- 1+
- 2−
- 2+
- 1−
4.
- cation
- anion
- neither
- cation
- neither
- neither
5.
- neither
- anion
- anion
- cation
- neither
- neither
6.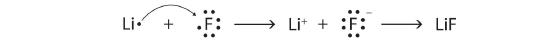
7. 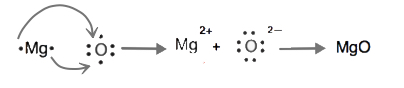
8. 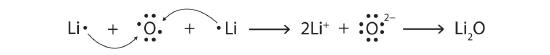
9. 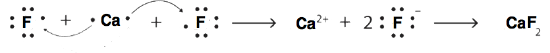
10. 1+
11. 2+
12. 2−
13. 1−


