2.4: Atomic Weight
- Page ID
- 429972
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Define atomic weight and atomic mass unit.
Even though atoms are very tiny pieces of matter, they have mass. Their masses are so small, however, that chemists often use a unit other than grams to express them. Is the the atomic mass unit (amu).
Atomic Mass Unit
The atomic mass unit (abbreviated amu) is defined as 1/12 of the mass of a 12C atom:
\[\mathrm{1\:amu=\dfrac{1}{12}\textrm{ the mass of }^{12}C\:atom} \label{Eq1} \]
It is equal to 1.661 × 10−24 g.
Masses of other atoms are expressed with respect to the atomic mass unit. For example, the mass of an atom of 1H is 1.008 amu, the mass of an atom of 16O is 15.995 amu, and the mass of an atom of 32S is 31.97 amu. Note, however, that these masses are for particular isotopes of each element. Because most elements exist in nature as a mixture of isotopes, any sample of an element will actually be a mixture of atoms having slightly different masses (because neutrons have a significant effect on an atom’s mass). How, then, do we describe the mass of a given element? By calculating an average of an element’s atomic masses, weighted by the natural abundance of each isotope, we obtain a weighted average mass called the atomic mass (also commonly referred to as the atomic weight) of an element.
Atomic Weight is the Weighted Average Mass of Isotopes
As stated above, most elements occur naturally as a mixture of two or more isotopes. Listed below (Table \(\PageIndex{1}\)) are the naturally occurring isotopes of selected elements along with the percent natural abundance of each.
| Element | Isotope (Symbol) | Percent Natural Abundance | Atomic Mass \(\left( \text{amu} \right)\) | Average Atomic Mass \(\left( \text{amu} \right)\) |
|---|---|---|---|---|
| Hydrogen | \(\ce{_1^1H}\) | 99.985 | 1.0078 | 1.0079 |
| \(\ce{_1^2H}\) | 0.015 | 2.0141 | ||
| \(\ce{_1^3H}\) | negligible | 3.0160 | ||
| Carbon | \(\ce{_6^{12}C}\) | 98.89 | 12.000 | 12.011 |
| \(\ce{_6^{13}C}\) | 1.11 | 13.003 | ||
| \(\ce{_6^{14}C}\) | trace | 14.003 | ||
| Oxygen | \(\ce{_8^{16}O}\) | 99.759 | 15.995 | 15.999 |
| \(\ce{_8^{17}O}\) | 0.037 | 16.995 | ||
| \(\ce{_8^{18}O}\) | 0.204 | 17.999 | ||
| Chlorine | \(\ce{_{17}^{35}Cl}\) | 75.77 | 34.969 | 35.453 |
| \(\ce{_{17}^{38}Cl}\) | 24.23 | 36.966 | ||
| Copper | \(\ce{_{29}^{63}Cu}\) | 69.17 | 62.930 | 63.546 |
| \(\ce{_{29}^{65}Cu}\) | 30.83 | 64.928 |
For some elements, one particular isotope is much more abundant than any other isotopes. For example, naturally occurring hydrogen is nearly all hydrogen-1, and naturally occurring oxygen is nearly all oxygen-16. For many other elements, however, more than one isotope may exist in substantial quantities. Chlorine (atomic number 17) is yellowish-green toxic gas. About three quarters of all chlorine atoms have 18 neutrons, giving those atoms a mass number of 35. About one quarter of all chlorine atoms have 20 neutrons, giving those atoms a mass number of 37. Were you to simply calculate the arithmetic average of the precise atomic masses, you would get approximately 36.
\[\frac{34.969 \,amu + 36.966 \,amu}{2} = 35.968 \,amu \nonumber \]
As you can see, the average atomic mass (atomic weight) given in the last column of the table above (35.453) is significantly lower. Why? The reason is that we need to take into account the natural abundance percentages of each isotope in order to calculate what is called the weighted average. The atomic weight of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element.
\[0.7577 \left( 34.969 \,amu \right) + 0.2423 \left( 36.966 \,amu \right) = 35.453 \,amu \nonumber \]
The weighted average is determined by multiplying the percent of natural abundance by the actual mass of the isotope. This is repeated until there is a term for each isotope. For chlorine, there are only two naturally occurring isotopes so there are only two terms.
Atomic weight = (%1)(mass 1) + (%2)(mass 2) + ⋯
Another example: oxygen exists as a mixture that is 99.759% 16O, 0.037% 17O and 0.204% 18O. The atomic weight of oxygen (use percent natural abundance data from Table 2.5.1) would be calculated as follows:
Atomic weight = (%1)(mass 1) + (%2)(mass 2) + (%3)(mass 3)
\[0.99759 \left( 15.995 amu \right) + 0.00037 \left( 16.995 amu \right) +0.00204 \left( 17.999 amu \right)= 15.999 amu \nonumber \]
To confirm your answer, compare the calculated value to the weighted mass displayed on the periodic table.
Calculate the atomic weight of oxygen. Oxygen exists as a mixture of 3 isotopes. Their respective masses and natural abundance are shown below.
- 16O: 15.995 amu (99.759%)
- 17O: 16.995 amu (0.037%)
- 18O: 17.999 amu (0.204%)
Solution
Multiply the isotope abundance by the actual mass of the isotope, and then sum up the products.
\[0.99759 \left( 15.995\, amu \right) + 0.00037 \left( 16.995 \,amu \right) +0.00204 \left( 17.999\, amu \right)= 15.999\, amu \nonumber \]
Exercise \(\PageIndex{1}\)
Calculate the atomic weight of copper. Copper exists as a mixture of 2 isotopes. Their respective masses and natural abundance are shown below.
- 63Cu: 62.930 amu (69.17%)
- 65Cu: 64.928 amu (30.83%)
- Answer
-
63.546 amu
The atomic weight of each element is found under the element symbol in the periodic table. Examples are shown below. The atomic weight of tin (Sn) is 118.71 amu while the atomic weight of carbon (C) is 12.011 amu. On the other hand, the atomic number (Z) of each element is found above the atomic symbol.
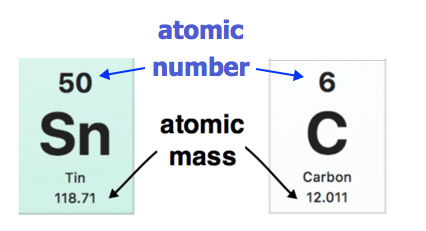
Atomic Weight indicated on entries of the Periodic Table. (public Domain; Pubchem)
Concept Review Exercises
- Define atomic weight. Why is it considered a weighted average?
- What is an atomic mass unit?
Answers
- The atomic weight is an average of an element’s atomic masses, weighted by the natural abundance of each isotope of that element. It is a weighted average because different isotopes have different masses.
- An atomic mass unit is 1/12th of the mass of a 12C atom.
Key Takeaway
- Atoms have a mass that is based largely on the number of protons and neutrons in their nucleus.
- The atomic weight of each element in the Periodic Table is the weighted average of the mass of all its isotopes.


