12.2: Preparation of Alkynes - Elimination Reactions of Dihalides
- Page ID
- 418169
After completing this section, you should be able to
- write an equation to describe the preparation of an alkyne by the dehydrohalogenation of a vicinal dihalide or vinylic halide.
- identify the alkyne produced from the dehydrohalogenation of a given vicinal dihalide or vinylic halide.
- write a reaction sequence to show how the double bond of an alkene can be transformed into a triple bond.
- identify the vicinal dihalide (or vinylic halide) needed to synthesize a given alkyne by dehydrohalogenation.
Make certain that you can define, and use in context, the key terms below.
- vicinal dihalide
- vinylic halide
Alkynes can be a useful functional group to synthesize due to some of their antibacterial, antiparasitic, and antifungal properties. One simple method for alkyne synthesis is by double elimination from a dihaloalkane.
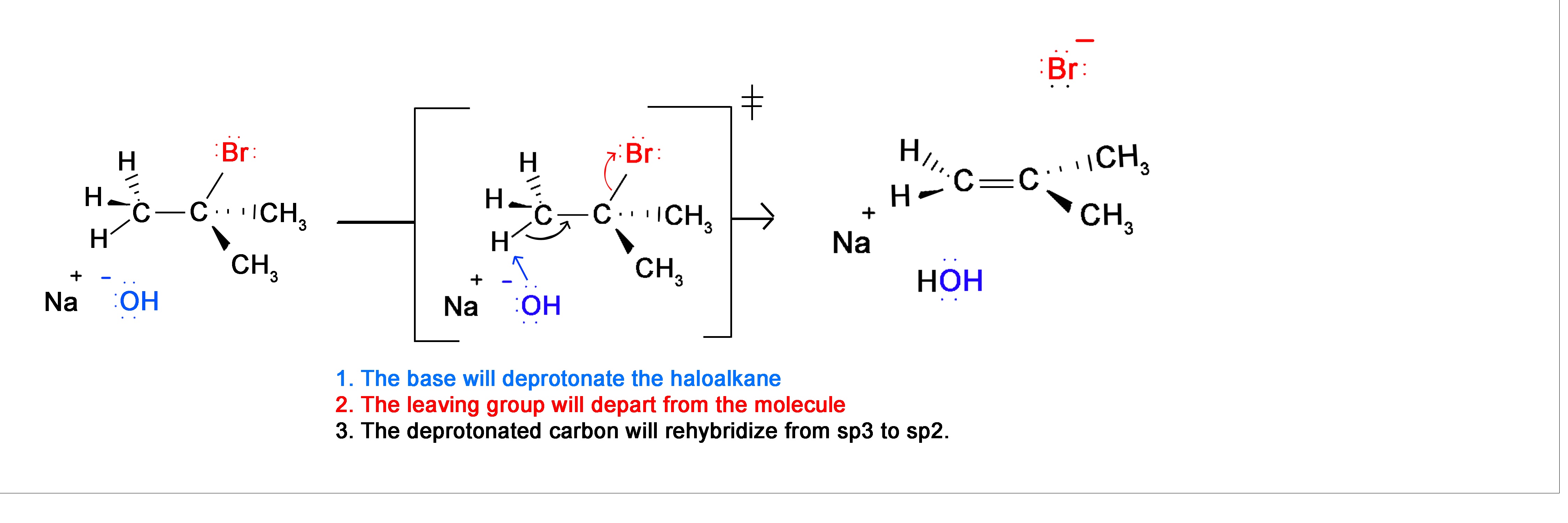
E2 Mechanism
Alkenes can be formed through an elimination reaction. In particular, the synthesis of alkynes will utilize the E2 elimination reaction. During the mechanism of an E2 reaction, a strong base removes a hydrogen adjacent to a halogen. The electrons from the broken C-H bond move to form the C=C double bond. Doing this causes the halogen to be ejected from the compound. Overall, a hydrogen and a halogen are eliminated from the compound to form an alkene. During this mechanism there is a stereoelectronic requirement that the adjacent hydrogen and the halogen be adjacent to each other.
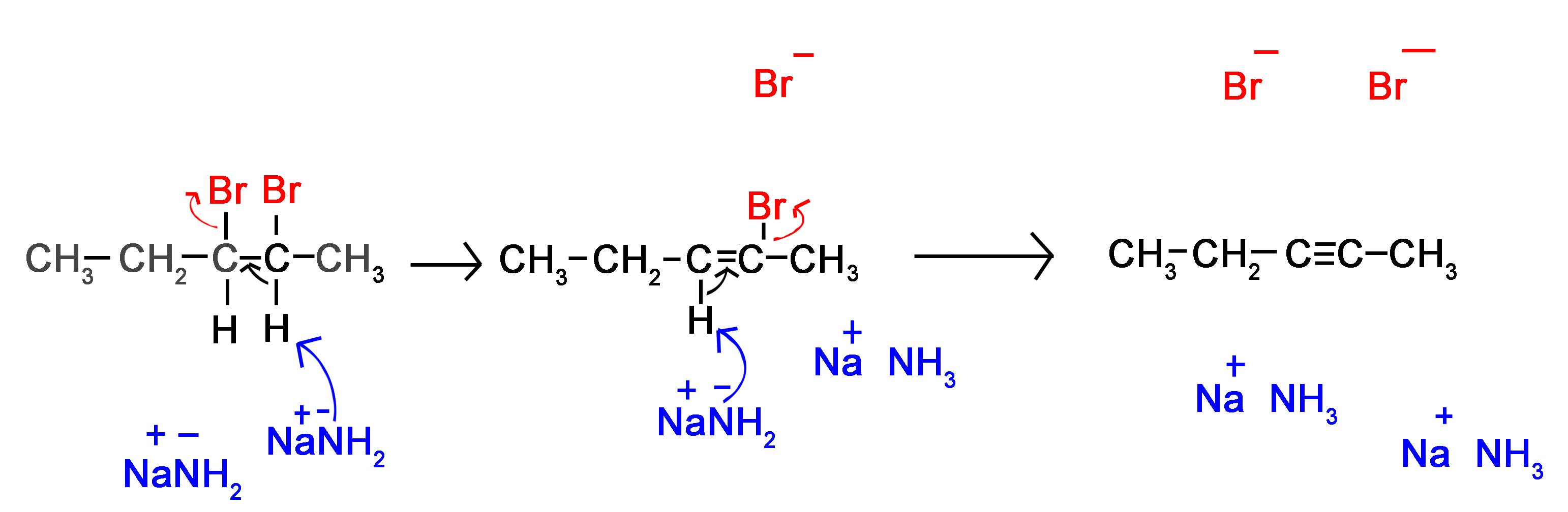
Alkyne Formation Through Dihaloalkane Elimination
Alkynes are frequently prepared through a double E2 reaction using 2 halides that are vicinal (meaning on adjacent carbons) or geminal (meaning on the same carbon). Because the E2 reaction takes place twice 2 \(\pi\) bonds are formed thus creating an Alkyne. Although hydroxide and alkoxide bases could be used for the strong base required for an E2 reaction, their used opens the possibility of position rearrangement in the alkyne product. Because of this, the stronger base sodium amide in ammonia (NaNH2/NH3) is commonly used.
General Reaction
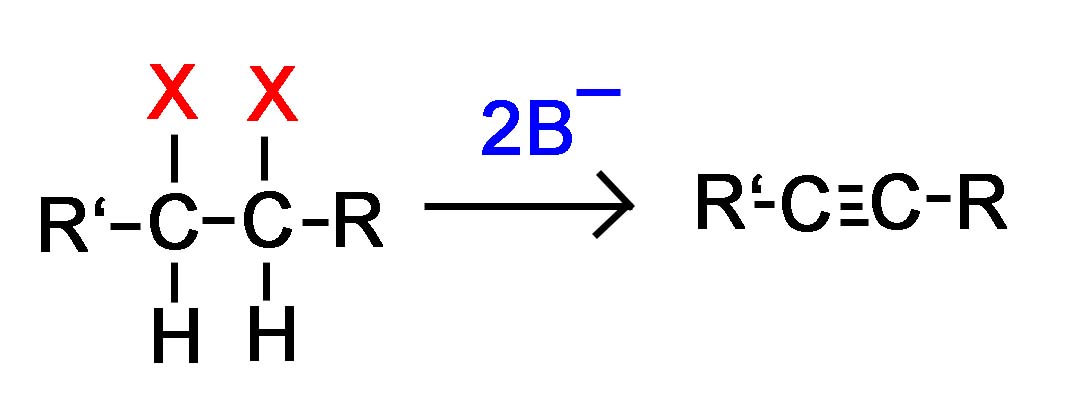
Vicinal dihalide converted to an alkyne
or
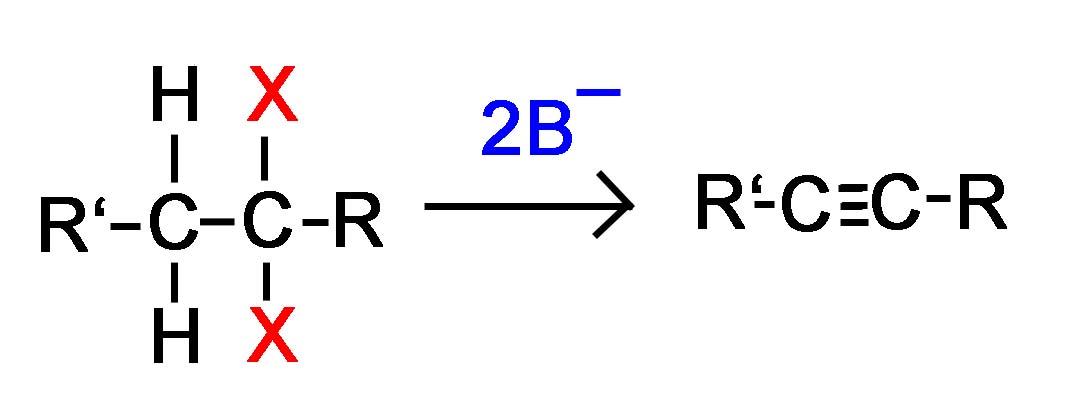
Geminal dihalide converted to an alkyne
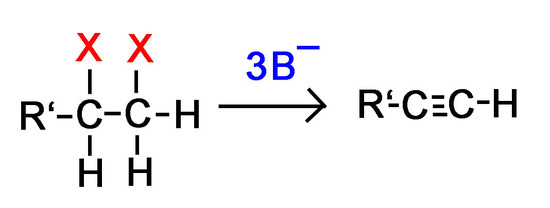
Note! If a terminal alkyne is formed during the reaction, 3 equivalents of base are required instead of 2 as discussed below.
Mechanism
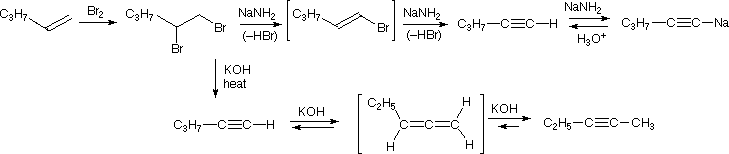
The following mechanism represents the reaction between 2,3-Dibromopentane with sodium amide in liquid ammonia to form pent-2-yne. During this mechanism an intermediate alkene is formed. Notice that in the alkene intermediate, the remaining hydrogen and halogen are anti to each other due to the stereoelectronic requirements of the E2 mechanism. The intermediate alkene is converted to an alkyne by a second E2 elimination of a hydrogen and halogen.
Terminal Alkynes
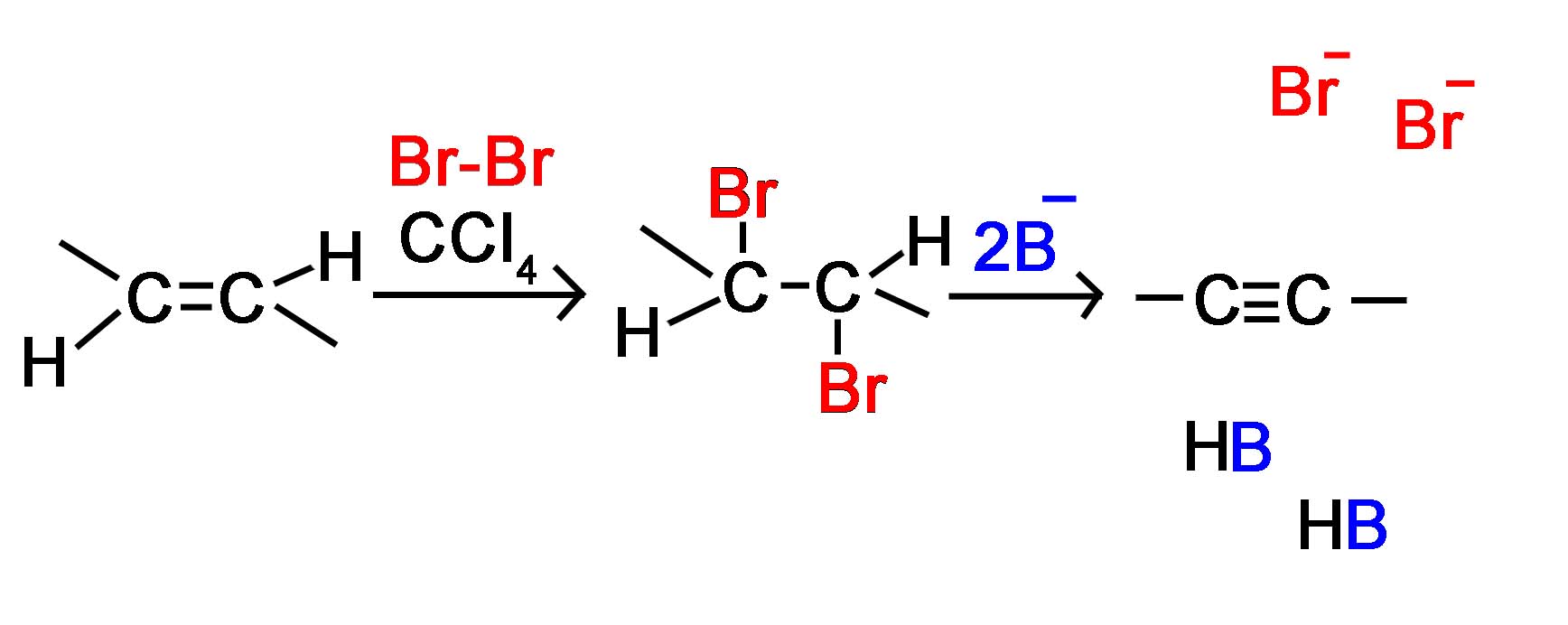
The acidity of terminal alkynes also plays a role in product determination when vicinal (or geminal) dihalides undergo base induced dielimination reactions. The following example illustrates eliminations of this kind starting from 1,2-dibromopentane, prepared from 1-pentene by addition of bromine. The initial elimination presumably forms 1-bromo-1-pentene, since base attack at the more acidic and less hindered 1º-carbon should be favored. The second elimination then produces 1-pentyne. If the very strong base such as sodium amide is used, the terminal alkyne is trapped as its sodium salt, from which it may be released by mild acid treatment. However, if the weaker base KOH with heat is used for the elimination, the terminal alkyne salt is not formed, or is formed reversibly, and the initially generated 1-pentyne rearranges to the more stable 2-pentyne via an allene intermediate. Even though terminal alkynes can be generated using sodium amide as a base, most chemists will prefer to use SN2 nucleophilic substitution instead of elimination when trying to form a terminal alkyne.
Preparation of Alkynes from Alkenes
An simple method for the preparation of alkynes utilizes alkenes as starting material. The process begins with the electrophilic addition of a halogen to the alkene bond to form the dihaloalkane. Then the double E2 elimination process is used to form the 2 \(\pi\) bonds of an alkyne.
This first process is gone over in much greater detail in the page on halogenation of an alkene. In general, chlorine or bromine is used with an inert halogenated solvent like chloromethane to create a vicinal dihalide from an alkene. The vicinal dihalide formed is the reactant needed to produce the alkyene using double elimination, as covered previously on this page.
1) Why would we need 3 bases for every terminal dihaloalkane instead of 2 in order to form an alkyne?
2) What are the major products of the following reactions:
a.) 1,2-Dibromopentane with sodium amide in liquid ammonia
b.) 1-Pentene first with Br2 and chloromethane, followed by sodium ethoxide (Na+ -O-CH2CH3)
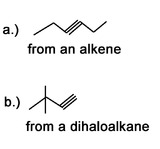
3) What would be good starting molecules for the synthesis of the following molecules:
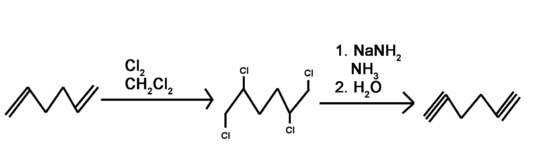
4) Use a 6 carbon diene to synthesize a 6 carbon molecule with 2 terminal alkynes.
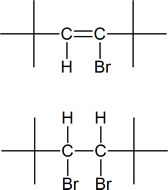
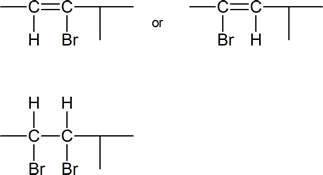
5) Identify the vinyl halide or halides and the vicinal dihalide or dihalides that could be used in the synthesis of:
a) 2,2,5,5-Tetramethyl-3-hexyne.
b) 4-Methyl-2-hexyne.
- Answer
-
1) Remember that hydrogen atoms on terminal alkynes make the alkyne acidic. One of the base molecules will pull off the terminal hydrogen instead of one of the halides like we want.
2)
a) 1-Pentyne
b) 1-Pentyne
3)
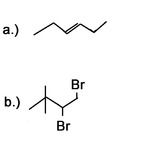
4) Bromine or chlorine can be used with different inert solvents for the halogenation. This can be done using many different bases. Liquid ammonia is used as a solvent and needs to be followed by an aqueous work-up.
5)
a)
b)
References
- Vollhardt, Peter, and Neil Shore. Organic Chemistry: Structure and Function. 5th. New York: W.H. Freeman and Company, 2007.
- Daley, Richard, and Sally Daley. "13.8 Elimination of Organohalogens." Organic Chemistry. Daley. 5 July 2005. 21 Feb. 2009. <https://studylib.net/doc/8721401/13-elimination-reactions>.