11.8: Oxidation of Alkenes to Vicinal Diols
- Page ID
- 418159
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- write the equation for the hydroxylation of an alkene using osmium tetroxide, and draw the structure of the cyclic intermediate.
- draw the structure of the diol formed from the reaction of a given alkene with osmium tetroxide.
- identify the alkene, the reagents, or both, that must be used to prepare a given 1,2-diol.
Make certain that you can define, and use in context, the key terms below.
- diol
- glycol
- hydroxylation
The previous section discussed the reduction of a double bond, so adding hydrogen to the the double bond. This section will discuss oxidation. In organic chemistry, this is a reaction that where the carbon atom loses electron density, which happens when new bond to a more electronegative atom occurs or when a double bond is broken between a carbon and a less electronegative atom. A simplified to say this is in organic chemistry a reduction is more bonds to hydrogen and oxidation is more bonds to oxygen often.
Syn Dihydroxylation
A glycol, also known as a vicinal diol, is a compound with two -OH groups on adjacent carbons. Osmium tetroxide oxidizes alkenes to give glycols through a syn addition, resulting in cis vicinal diols. Next semester in the chapter on ethers, you'll learn how to make trans vicinal diols.
Introduction
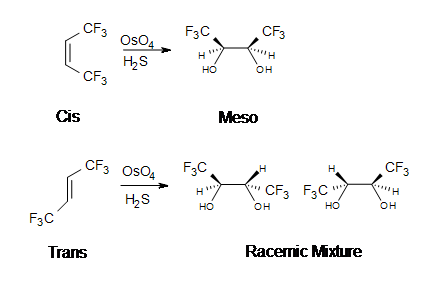
The reaction with \(OsO_4\) is a concerted process that has a cyclic intermediate and no rearrangements. When an alkene reacts with osmium tetroxide, stereocenters can form in the glycol product. Cis alkenes give meso products and trans alkenes give racemic mixtures.
\(OsO_4\) is formed slowly when osmium powder reacts with gasoues \(O_2\) at ambient temperature. Reaction of bulk solid requires heating to 400 °C:
\[Os_{(s)} + 2O_{2\;(g)} \rightarrow OS_4\]
Since Osmium tetroxide is expensive and highly toxic, the reaction with alkenes has been modified. Catalytic amounts of OsO4 and stoichiometric amounts of an oxidizing agent such as hydrogen peroxide are now used to eliminate some hazards. Also, an older reagent that was used instead of OsO4 was potassium permanganate, \(KMnO_4\). Although syn diols will result from the reaction of KMnO4 and an alkene, potassium permanganate is less useful since it gives poor yields of the product because of overoxidation.
Mechanism
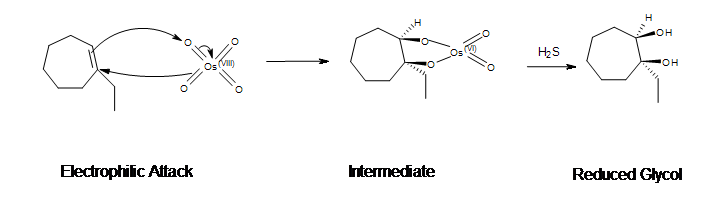
- Electrophilic attack on the alkene
- Pi bond of the alkene acts as the nucleophile and reacts with osmium (VIII) tetroxide (OsO4)
- 2 electrons from the double bond flows toward the osmium metal
- In the process, 3 electron pairs move simultaneously
- Cyclic ester with Os (VI) is produced
- Reduction
- H2S reduces the cyclic ester
- NaHSO4 with H2O may be used
- Forms the syn-1,2-diol (glycol)
- H2S reduces the cyclic ester
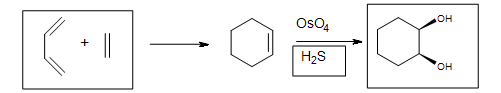
Example: Dihydroxylation of 1-ethyl-1-cycloheptene
Hydroxylation of alkenes
Dihydroxylated products (glycols) are obtained by reaction with aqueous potassium permanganate (pH > 8) or osmium tetroxide in pyridine solution. Both reactions appear to proceed by the same mechanism (shown below); the metallocyclic intermediate may be isolated in the osmium reaction. In basic solution the purple permanganate anion is reduced to the green manganate ion, providing a nice color test for the double bond functional group. From the mechanism shown here we would expect syn-stereoselectivity in the bonding to oxygen, and regioselectivity is not an issue.
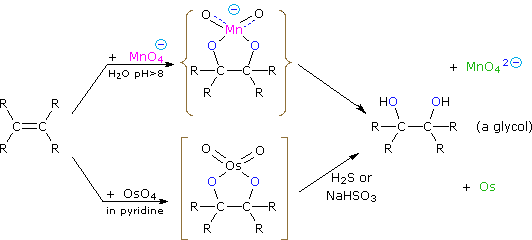
When viewed in context with the previously discussed addition reactions, the hydroxylation reaction might seem implausible. Permanganate and osmium tetroxide have similar configurations, in which the metal atom occupies the center of a tetrahedral grouping of negatively charged oxygen atoms. How, then, would such a species interact with the nucleophilic pi-electrons of a double bond? A possible explanation is that an empty d-orbital of the electrophilic metal atom extends well beyond the surrounding oxygen atoms and initiates electron transfer from the double bond to the metal, in much the same fashion noted above for platinum. Back-bonding of the nucleophilic oxygens to the antibonding π*-orbital completes this interaction. The result is formation of a metallocyclic intermediate, as shown above.
Outside links
References
- Dehestani, Ahmad et al. (2005). Ligand-assisted reduction of osmium tetroxide with molecular hydrogen via a [3+2] mechanism. Journal of the American Chemical Society, 2005, 127 (10), 3423-3432.
- Sorrell, Thomas, N. Organic Chemistry. New York: University Science Books, 2006.
- Vollhardt, Peter, and Neil E. Schore. Organic Chemistry: Structure and Function. 5th Edition. New York: W. H. Freeman & Company, 2007.
1. Give the major product.
.bmp?revision=1&size=bestfit&width=131&height=51)
2. What is the product in the dihydroxylation of (Z)-3-hexene?

3. What is the product in the dihydroxylation of (E)-3-hexene?

4. Draw the intermediate of this reaction.

5. Fill in the missing reactants, reagents, and product.
- Answers
-
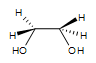
1. A syn-1,2-ethanediol is formed. There is no stereocenter in this particular reaction. The OH groups are on the same side.
2. Meso-3,4-hexanediol is formed. There are 2 stereocenters in this reaction.
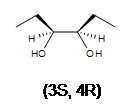
3. A racemic mixture of 3,4-hexanediol is formed. There are 2 stereocenters in both products.

4. A cyclic osmic ester is formed.
5. The Diels-Alder cycloaddition reaction is needed in the first box to form the cyclohexene. The second box needs a reagent to reduce the intermediate cyclic ester (not shown). The third box has the product: 1,2-cyclohexanediol.
Contributors and Attributions
- Shivam Nand
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
- Kristen Perano
- Layne Morsch (University of Illinois Springfield)
- Lauren Reutenauer (Amherst College)

