Nuclear Weapons
- Page ID
- 1472
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)A nuclear weapon is commonly defined as a device, which uses a nuclear reaction for destructive means. The first nuclear weapon was successfully detonated on July 16, 1945. The nuclear weapon, code named “Trinity”, yielded an explosion which was equivalent to 20 kilotons of Trinitrotoluene (TNT). This reaction unexpectedly had a shock wave which could be felt 100 miles away. Compared to chemical reactions, the amount of energy that can be released from nuclear reactions can be up to a million times greater. Before we can fully understand the chemical complexity and appreciate the engineering elegance of a nuclear weapon, however, it is important to grasp basic nuclear chemistry concepts.
Nuclear Fission
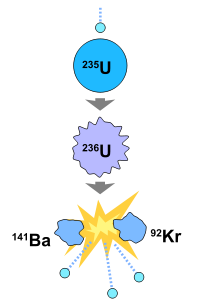
This type of nuclear reaction is caused by nuclear decay of an unstable atom that has been hit by a neutron. As a result of the instability of the atom, the nucleus splits into two fission fragments also yielding free neutrons and exorbitant amounts of energy (both in the form of electromagnetic radiation and kinetic energy). A neutron carries no electric charge but the nucleus of an atom does - a positive charge. Like with magnets, like charges repel each other, so particles that carry positive electric charges like alpha particles are repelled by the nucleus of an atom and thus do not stick to the nucleus. However, a neutrally charged neutron can combine with the nucleus of an atom which then causes the nucleus to become unstable and split into 2 nuclei. According to Einstein's formula, \(E=mc^2\), energy is released from this reaction along with other neutrons that have the same effect on nearby nuclei and a chain reaction occurs which yields an incredible amount of energy.
Nuclear Fusion
Fusion is almost completely complimentary to fission. It is the process in which a nuclear reaction where two nuclei are joined together to form a heavier nucleus. The reaction also yields free neutrons and exorbitant amounts of energy from binding energy. Our sun is a "nuclear furnace" in the sense that it is a place where nuclear fusion occurs. Although a nuclear fusion reactor would undoubtedly be better for our environment, such reactions are not yet possible because no known material can withstand the incredible high temperatures needed for such reactions to occur.
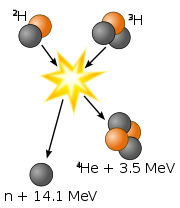
Example of Fusion Reaction
\[\ce{^2D+^3T → ^4_2He +^1_0N} + 17.6\, MeV\]
- Fissile / Fissionable An atom is fissionable if it is capable of undergoing fission. If an atom is Fissile it is not only able to undergo fission, but it is also capable of sustaining a nuclear chain reaction.
- Nuclear Chain Reaction: As stated earlier in the text, a nuclear fission reaction yields free neutrons. In a nuclear chain reaction, the free neutrons from a nuclear fission reaction bombard nearby Fissile isotopes resulting in multiple fission reactions. These reactions result in colossal amounts of kinetic energy and gamma radiation.
- Critical Mass: The amount of fissile isotope required to successfully assist a nuclear chain reaction. If the fissile material is at a subcritical mass it cannot sustain a nuclear chain reaction. On the other hand, if the fissile material is at a supercritical mass, it will undergo a chain reaction at a faster rate.
Nuclear Weaponry
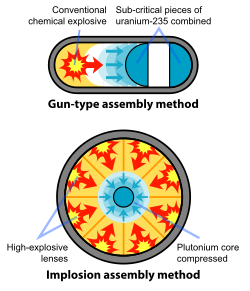
A nuclear weapon can either undergo a nuclear fission reaction (atomic bomb) or a nuclear fusion reaction (H bomb or thermonuclear bomb). The first nuclear weapons built underwent pure nuclear fission. Uranium-235 and Plutonium-239 were the most common fissile isotopes used. (Uranium-235 is less than 1% naturally abundant. It requires complex methods of enrichment to be a fissile isotope). There are 2 basic nuclear fission weapon designs:
- Gun assembly- An uranium-235 bullet is fired through a barrel at a fissile Uranium-235 target. The collision of the two isotopes initiates a chain reaction. The gun assembly design only proves practical with the uranium-235 isotope.
- Implosion- A critical mass of a fissile material (U-235 or Pu-239) is surrounded by highly explosive material. When detonated, the high explosives compress the fissile material causing it to assume a state of supercritical mass. The supercritical mass instantaneously begins to undergo a chain reaction.
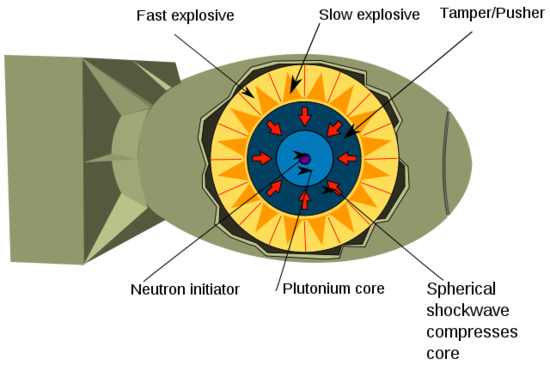
Weapons Utilizing Fusion Reactions
The first nuclear fusion weapons (also known as thermonuclear weapons) were designed to initiate a fission-based chain reaction. The fusion reaction between tritium and deuterium would result in the free neutrons necessary to bombard a fissile isotope and start a nuclear chain reaction. The first thermonuclear weapon was detonated in November of 1952. Similar to the nuclear implosion design, thermonuclear weapons use the heat and radiation from a fission reaction to make the fissile material assume a state of supercritical mass. The supercritical mass then instantaneously undergoes a fusion chain reaction yielding exponentially more energy than a fission chain reaction.
References
- Miller, Richard L. Under the Cloud : The Decades of Nuclear Testing. New York: Free P, 1999.
- Petrucci, Ralph H., William S. Harwood, and Geoff E. Herring. General Chemistry : Principles and Modern Applications. Upper Saddle River: Prentice Hall PTR, 2006. 1041-065.
Exercise
1. Given the following nuclear fusion reaction, what particle corresponds to \(\ce{^{?}_{?}X}\)?
\[\ce{^2_1H + ^2_1H \rightarrow ^{?}_{?}X + \rm{energy}}\]
- \(\ce{^4_2He}\)
- \(\ce{^{175}_{87}Fr}\)
- \(\ce{^{14}_6C}\)
- \(\ce{^0_{-1}e}\)
- none of the above
2. Which of the following best describes a Nuclear Fusion Reaction?
- A nuclear reaction in which 2 small nuclei combine to form one large nucleus.
- A reaction in which a large nucleus splits into two smaller and fast moving particles.
- The combination of a nucleus and an electron.
- The combination of a nucleus and a positron.
- The combination of a nucleus and an alpha particle?
3. Which of the following facts describes a Nuclear Fission Reaction?
- A nuclear reaction in which 2 small nuclei combine to form one large nucleus.
4. The following nuclear reaction is carried out with the corresponding masses of the reactants:
31 H + 11 H yields 42 He + Energy
7.556g 2.143g Xg
If 4.56 X1011 Joules of energy was released, what mass in grams of 42 He is produced?
- A reaction in which a large nucleus splits into two smaller and fast moving particles.
- The combination of a nucleus and an electron.
- The combination of a nucleus and a positron.

