Terpenes
- Page ID
- 5876
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Compounds classified as terpenes constitute what is arguably the largest and most diverse class of natural products. A majority of these compounds are found only in plants, but some of the larger and more complex terpenes (e.g. squalene & lanosterol) occur in animals. Terpenes incorporating most of the common functional groups are known, so this does not provide a useful means of classification. Instead, the number and structural organization of carbons is a definitive characteristic. Terpenes may be considered to be made up of isoprene (more accurately isopentane) units, an empirical feature known as the isoprene rule. Because of this, terpenes usually have \(5n\) carbon atoms (\(n\) is an integer), and are subdivided as follows:
| Classification | Isoprene Units | Carbon Atoms |
|---|---|---|
| monoterpenes | 2 | C10 |
| sesquiterpenes | 3 | C15 |
| diterpenes | 4 | C20 |
| sesterterpenes | 5 | C25 |
| triterpenes | 6 | C30 |
Isoprene itself, a C5H8 gaseous hydrocarbon, is emitted by the leaves of various plants as a natural byproduct of plant metabolism. Next to methane it is the most common volatile organic compound found in the atmosphere. Examples of C10 and higher terpenes, representing the four most common classes are shown in the following diagrams. Most terpenes may be structurally dissected into isopentane segments. How this is done can be seen in the diagram directly below.
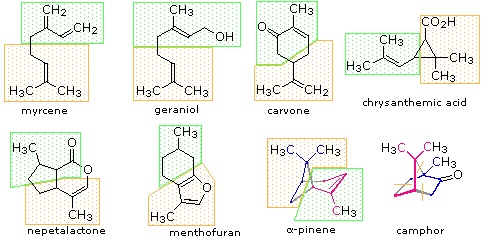
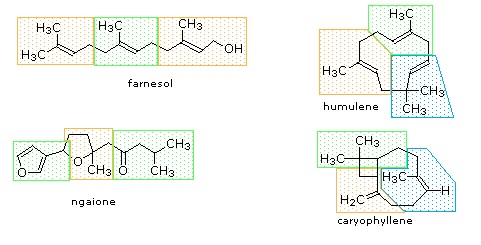
Figure: Monoterpenes and diterpenes
The isopentane units in most of these terpenes are easy to discern, and are defined by the shaded areas. In the case of the monoterpene camphor, the units overlap to such a degree it is easier to distinguish them by coloring the carbon chains. This is also done for alpha-pinene. In the case of the triterpene lanosterol we see an interesting deviation from the isoprene rule. This thirty carbon compound is clearly a terpene, and four of the six isopentane units can be identified. However, the ten carbons in center of the molecule cannot be dissected in this manner. Evidence exists that the two methyl groups circled in magenta and light blue have moved from their original isoprenoid locations (marked by small circles of the same color) to their present location. This rearrangement is described in the biosynthesis section. Similar alkyl group rearrangements account for other terpenes that do not strictly follow the isoprene rule.
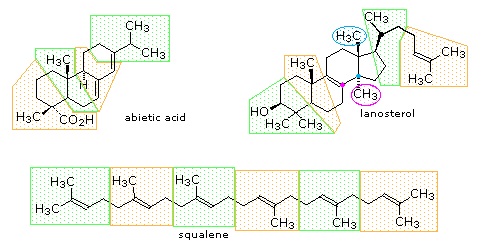
Figure: Triterpenes
Polymeric isoprenoid hydrocarbons have also been identified. Rubber is undoubtedly the best known and most widely used compound of this kind. It occurs as a colloidal suspension called latex in a number of plants, ranging from the dandelion to the rubber tree (Hevea brasiliensis). Rubber is a polyene, and exhibits all the expected reactions of the C=C function. Bromine, hydrogen chloride and hydrogen all add with a stoichiometry of one molar equivalent per isoprene unit. Ozonolysis of rubber generates a mixture of levulinic acid ( \(CH_3COCH_2CH_2CO_2H\) ) and the corresponding aldehyde. Pyrolysis of rubber produces the diene isoprene along with other products.
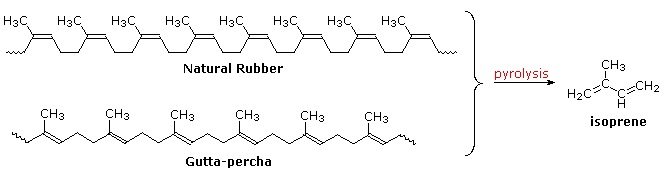
The double bonds in rubber all have a Z-configuration, which causes this macromolecule to adopt a kinked or coiled conformation. This is reflected in the physical properties of rubber. Despite its high molecular weight (about one million), crude latex rubber is a soft, sticky, elastic substance. Chemical modification of this material is normal for commercial applications. Gutta-percha (structure above) is a naturally occurring E-isomer of rubber. Here the hydrocarbon chains adopt a uniform zig-zag or rod like conformation, which produces a more rigid and tough substance. Uses of gutta-percha include electrical insulation and the covering of golf balls.
Contributors
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry


