Set 2 – Energy Transition and Population
- Page ID
- 79416
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)What frequency of electromagnetic radiation is needed to excite a nuclear spin flip?
Students will not know the answer to this unless (1) they remember it from coverage of NMR spectroscopy in a prior organic course or (2) they know something about the NMR spectrometer in the department and can reason out that the MHz designation is a frequency that occurs in the RF part of the spectrum (prompting them with the department’s spectrometer is a way to get them to consider this.
Where is radiofrequency (RF) radiation on the energy scale of the electromagnetic spectrum?
Groups usually know that RF radiation is at the low energy end of the spectrum, especially if this unit is being done in a course where other spectroscopic methods have been developed.
I then set up the next question by asking the students to think about a valence electron \(\pi\)-\(\pi\)* transition that occurs in the UV part of the spectrum compared to a nuclear spin-flip in the RF region. That allows us to draw an energy level diagram like that shown in Figure 4 in the text, although without showing the populations of the levels. I further point out the idea that there is thermal energy and ask them the following question.
Is the thermal energy at room temperature large or small compared to the energy of a \(\pi\)-\(\pi\)* transition and to the energy of a nuclear spin flip? What are the consequences of your answers to these questions?
Students can rationalize that thermal energy is insufficient to excite valence electrons but have a hunch that it is sufficient to excite nuclear spin flips given that they are being asked this question. When it comes to the consequences, they may be tempted to think that all the nuclei are excited. That leads to a discussion about how we can use the Boltzmann distribution to calculate the populations of the two levels. I then provide the example populations shown in Figure 4 in the text and ask them the following question.
If thermal energy has sufficient energy to excite nuclear spin flips, why are there still more in the ground than excited state?
They can reason out that because the ground state is lower in energy, chemical systems will have a preference for that state. But I make a point of emphasizing how the two populations are almost equal and that will have important consequences for us later with the sensitivity of NMR spectroscopy and with coupling.
Can you think of two processes by which a specific excited state nucleus can get rid of its excess energy?
Students’ ability to answer this may depend on when this unit is done in a course. If it is later in a course on spectroscopy, they are more likely to come up with the loss of energy to the surroundings as heat and spin-lattice relaxation. If they have had a prior unit on fluorescence or atomic emission they are likely to come up with the idea of spin-spin relaxation. There is also a good chance that some groups may need to be prompted on both of these processes by asking how other systems lose excess energy or by pointing out how excited state nuclei have exactly the same amount of extra energy as is needed to excite ground state nuclei.
Do excited state nuclei have short or long relaxation times?
Students can usually rationalize that the relaxation times are long because of the small energy gap and the small difference in population. That allows us to examine the change in population that occurs when a high power RF is applied as shown in Figure 5 in the text. They are then given the following question.
When the populations of the two levels are equal, can we continue to excite ground state nuclei up to the excited state such that the population of the excited state becomes larger than the population of the ground state, creating what is known as a population inversion?
Students’ ability to answer this will depend on whether they are in a course where lasers were previously discussed. If not, they likely will have no idea that it is impossible to have a population inversion in a 2-level system. If so, they may need to be reminded about what they learned previously when discussing how a laser worked.
I then either remind them or discuss the idea of absorption versus stimulated emission and use a diagram like that in Figure 6 in the text to show what will happen. This allows us to understand what is meant by a saturated transition.
I then explain that the observation that the populations of the two levels are almost equal is the reason NMR spectroscopy has low sensitivity. After that, I point out how the difference in energy between the ground and excited states depends on the magnitude of the applied magnetic field and show them Figure 7 in the text. Then they are given the following question.
What happens to the population distribution as the energy gap between the ground and excited state is increased?
Based on our prior discussion of the population differences between the two levels in a \(\pi\)-\(\pi\)* transition and nuclear spin transition they expect that the population difference will be larger the greater the energy gap. I then go over other things that can be done to improve the sensitivity of NMR spectroscopy: (1) use of microtubes, (2) use of a cryoprobe and (3) recording multiple spectra and adding them together.
I then present some additional equations for the energy gap between the ground and excited state that bring in the magnetogyric ratio and magnetic moment of the hydrogen nucleus (Equations 2-5 in the text), which raises the important distinction that the energy gap ultimately depends on the magnetic field experienced by the nucleus, which is different than the applied magnetic field. I describe how the magnetic field experienced by a nucleus is the sum of all the magnetic fields that exert a force on a particular nucleus.
Consider a sample in an NMR tube. The crosshatched region in the tube is the area over which signal is recorded. Why is it important that BAPPL be homogeneous over this entire region?
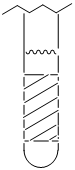
It may be necessary to prompt groups to look back at the equations and track how the applied magnetic field influences the experienced magnetic field, which influences the energy gap and then the frequency of excitation. When they realize that different molecules may have different frequencies of excitation, then you can ask them what this would do to the resulting resonance in the NMR spectrum.
I then go over why samples are spun when recording a spectrum. I also talk about the use of the deuterium signal in deuterated solvents as a means of tuning the spectrometer and locking on to a signal to account for any drift that may occur as spectra are recorded.


